As lithium-ion batteries continue to decrease in price, they are quickly replacing the lead-acid batteries traditionally used in cars and other vehicles. This is creating a sudden abundance of used lead-acid batteries, which would be harmful to the environment and people if not recycled properly. To help deal with this problem, researchers developed an environmentally friendly method to turn lead from used lead-acid batteries into photodetectors operating in the UV-visible band.
“We believe this recycling strategy could significantly reduce the lead pollution resulting from waste lead-acid batteries, which is important to the environment,” said research team leader Longxing Su from Southern University of Science and Technology in China. “The photodetectors promote the recycling economy by creating a market for recycled lead. They can be used for a variety of applications including optical communication, chemical analysis and imaging.”
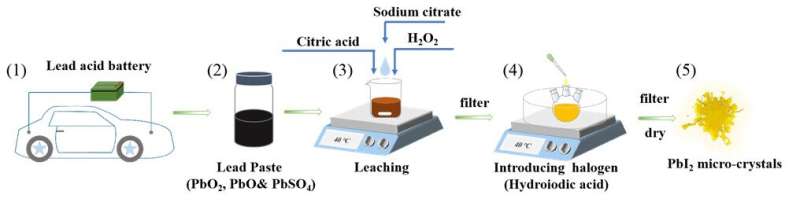
In the journal Optics Letters, Su and colleagues describe their process for extracting lead from discarded lead-acid batteries and then using it to synthesize lead(II)iodide (PbI2) microcrystals suitable for use in photodetectors.
“The recycled PbI2 microcrystals exhibit the quality and purity levels necessary for making photodetectors,” said Su. “We also show that the microcrystals can be used to make photodetectors with excellent stability, repeatability and fast response speeds.”
A new use for old batteries
Although the lead found in used lead-acid batteries can be recycled, most methods used for this are expensive and have various drawbacks. Su’s research team developed a more efficient strategy to produce PbI2 from the lead paste found within lead-acid batteries.
To extract the lead from the paste, the researchers developed a one-pot process that requires only inexpensive, easily obtained chemicals and no commercial precursors, which would increase cost. The process involves placing the paste into a mixed solution with excess citric acid monohydrate, sodium citrate dihydrate and H2O2. The excess sodium citrate dihydrate causes almost all the generated lead citrate to dissolve in the mixed solution. The mixture is then filtered to obtain a clear solution containing lead. When excess hydroiodic acid is added to the solution, it forms yellow PbI2 precipitate, which is collected and dried in a vacuum.
The researchers then used a simple spin-coating process to create a photodetector using the PbI2 produced through this process. They investigated the photo response of the photodetector using a 300 W Xe-lamp as a UV-Visible light source and a semiconductor parameter instrument as an electric signal collector. The PbI2 photodetector they fabricated showed a low dark current of 1.06 nA and an on-off ratio of 103 under 10 V bias voltage.
Next steps
The researchers are now working on scaling up their process to mass-produce recycled PbI2. Before commercialization, the recycled PbI2 and photodetectors made from it would need to be verified by independent testing organizations and companies interested in incorporating the photodetectors into downstream products.
“We hope that our work will be noticed by chemical companies and downstream firms so that our method can be extended into the market,” said Su. “Our green reactant recycling method may also be useful for other applications, such as making solar cells.”