Seeing is believing—or, rather, seeing can aid in understanding, especially when it comes to the mechanisms underpinning lithium-ion batteries. Despite near-ubiquitous use in cell phones, computers and more, the complex electrochemical environments of lithium-ion batteries remain murky.
To better understand and to improve battery performance, researchers examined the current scientific literature and used electron microscopy to take a closer look at the charge-transfer and lithium-ion migration mechanisms that produce power. This study was published in Nano Research Energy.
“Commercial lithium-ion batteries are widely used as energy storage devices, including electric vehicles, portable electronics and grid energy storage,” said Yi Ding, a professor of Tianjin University of Technology. “Energy, power, charge-discharge rate, cost, cycle life, safety and environmental impact are to be considered while adopting lithium-ion batteries for a suitable application, but each specific application faces a variety of different challenges.”
The amount of energy stored is important for portable electronics, while cost and safety are more important for electric vehicles, for example. Cost and safety are also important for energy grid needs, but energy density becomes less so than for electric vehicles. The trade-off between these elements shift based on need, but the ability to tune performance is limited by incomplete understanding of the materials used in batteries.
“The active electrode materials are the main portion responsible for the cell chemistry and performance and, ultimately, affect the commercialization of the constructed battery,” Ding said.
“The performances, such as cycle life and energy density, of existing commercial electrode material systems still need to be improved, so it is important to understand the inherent physical and chemical properties, such as structural evolution/kinetics during lithium de-embedding and the effect of electrode-electrolyte interface on the performance of lithium-ion batteries.”
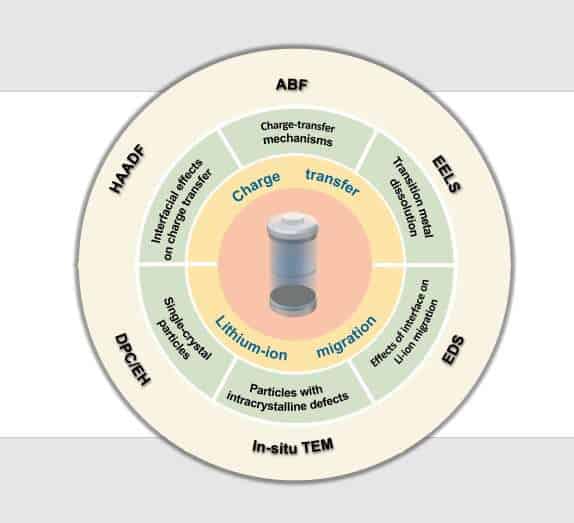
The researchers reviewed recent advances in electron microscopy to see how traditional characterization techniques measure up when it comes to understanding the structure-activity relationships of commercial lithium-ion batteries.
“By comparing with the characterization content obtained by traditional characterization techniques, such as X-ray diffraction and X-ray photoelectron spectroscopy, we illustrate the advantages and limitations of common electronic microscopes and recently developed advanced electronic microscopic characterization techniques, such as in situ electron microscopy technology, in this critical research,” Ding said.
The researchers examined how advanced electron microscopy and the associated characterization techniques can provide different insights into how, for example, lithium ions migrate in the battery to produce charge or how charge transfer can trigger energy use.
They specifically focused on transition metal dissolution and charge transfer mechanism in the charging-discharging process of lithium-ion battery positive electrodes; the structure and evolution of cathode-electrode interfaces and solid electrolyte interphase during long-term cycling; and the effect of electrode structure and interface on lithium-ion migration.
The conclusion, according to Ding, is that next-generation lithium-ion battery technologies with better cost and performance benefits are needed.
“We propose the possibility of combining electron microscopy with other techniques to obtain more comprehensive information,” Ding said, noting that electron microscopy has three common limitations in battery assessment.
These include inconsistent electrochemical environments between electron microscopy fields and actual batteries; unstable time windows that can skew data related to evolution of the sample; and certain batteries cannot be quantitatively assessed at the nanoscale. “Even with limitations, these discussions allow researchers to gain a deeper understanding of how commercial lithium-ion batteries operate at the microscale and provide guidance for design strategies for high-performance practical batteries,” the researchers noted.