Solar and wind are quickly transforming the energy landscape—but if we are to realize the full potential of these intermittent, renewable energy sources, we’ll need safe, affordable batteries capable of storing it.
As part of an effort to overcome the long-term energy-storage challenge, University of Wisconsin–Madison engineers have invented a water-soluble chemical additive that improves the performance of a type of electrochemical storage called a bromide aqueous flow battery.
“Bromide-based aqueous flow batteries are a promising solution, but there are many messy electrochemical problems with them. That’s why there’s no real successful bromide-based products today,” says Patrick Sullivan who graduated from UW–Madison with a Ph.D. in chemistry in 2023. “Yet, our one additive can solve so many different problems.”
Sullivan, Ph.D. student Gyohun Choi, and Dawei Feng, an assistant professor of materials science and engineering at UW–Madison, developed the additive. The research was published on October 23, 2024, by the journal Nature.
Currently, giant tractor-trailer-sized lithium-ion battery packs store energy for the grid—but with technical limitations. Lithium batteries have safety concerns due to the potential for fires and explosions and a complicated international supply chain.
Aqueous flow batteries, however, could make grid-scale storage safer and cheaper. In these batteries, positive and negative liquid electrolytes circulate over electrodes that are separated by a membrane. Since the batteries use ions dissolved in a liquid—water—they can be scalable, sustainable and safe.
The most commercially mature flow batteries are based on vanadium ions, which, like lithium, are expensive and hard to source. However, another version of these flow batteries relies on bromide, a cheap, widely available ion that performs similar to vanadium—at least on paper.
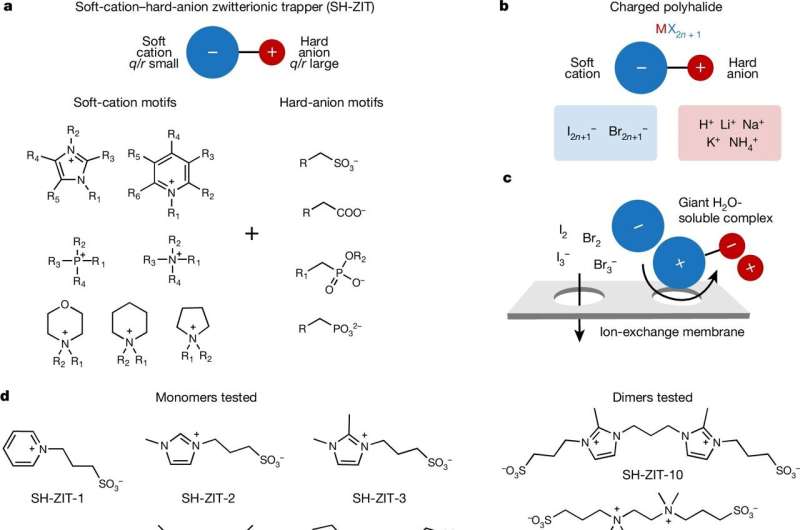
In practice, however, tiny bromide ions cause all sorts of problems in flow batteries. They can pass through the membrane that separates the electrodes, and that reduces the battery’s efficiency. Sometimes the ions precipitate out of the electrolyte and form a messy oil that “sinks” to the bottom of the solution. Occasionally, the ions also form toxic bromine gas. These issues hinder practical performance and reliability.
An additive called a complexing agent could help. Choi set out to find an additive that enhances bromide aqueous flow battery performance. The researchers used molecular design to engineer over 500 candidate organic molecules they call “soft-hard zwitterionic trappers.” They synthesized and tested 13 of these representative molecules as potential additives for the bromide batteries.
The resulting multi-functional additives solve the flow battery’s main problems. It encapsulates the bromide ions while allowing them to remain water-soluble, and since the resulting complex is now larger, they can’t pass through the membrane. The ions are also “phase-stable,” which means they don’t separate out of the water electrolyte or create toxic bromine gas.
Importantly, the additives dramatically improve the flow battery’s performance, increasing the efficiency and longevity of the chemical system. “Our devices with the additive functioned without decay for almost two months compared to ones without it, which typically fail within a day,” says Feng. “This is important because for green energy storage, you want to use it for 10 or 20 years.”
The team plans to continue refining the work. Choi will study the fundamental science behind additives for bromide and iodide flow batteries, while Sullivan, who is CEO of Flux XII—a renewable energy spinoff company he co-founded with Feng—will explore the commercial viability of the additive, which has already been successfully produced in industrial ton-scale reactions.