A sodium battery developed by researchers at The University of Texas at Austin significantly reduces fire risks from the technology, while also relying on inexpensive, abundant materials to serve as its building blocks.
Though battery fires are rare, increased battery usage means these incidents are on the rise.
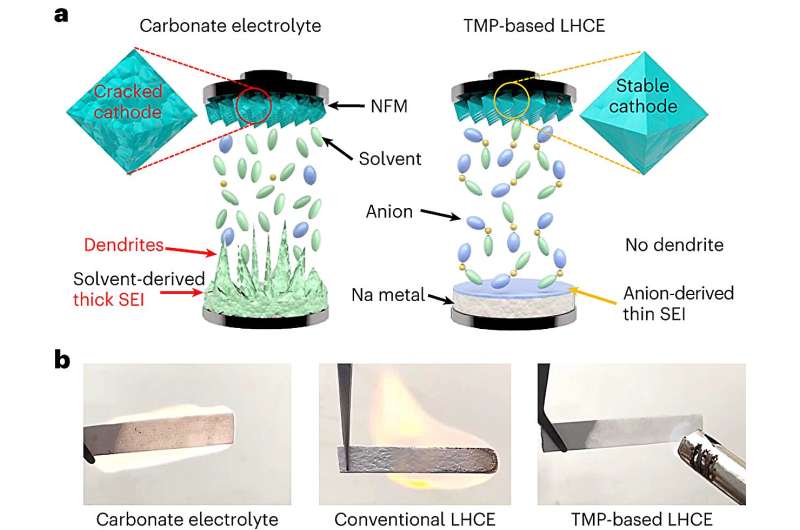
The secret ingredient to this sodium battery breakthrough, published recently in Nature Energy, is a solid diluent. The researchers used a salt-based solid diluent in the electrolyte, facilitating the charge-discharge cycle. A specific type of salt—sodium nitrate—allowed the researchers to deploy just a single, nonflammable solvent in the electrolyte, stabilizing the battery as a whole.
Over time, the multiple liquid solvents in an electrolyte—the component that transfers charge-carrying ions between the battery’s two electrodes—react with other components in ways that degrade batteries and lead to safety risks. Sodium, an alternative to lithium that is one of the key ingredients in this battery, is highly reactive, posing a significant challenge to the adoption of these types of batteries. These reactions can lead to the growth of needle-like filaments called dendrites that can cause the battery to electrically short and even catch fire or explode.
“Batteries catch fire because the liquid solvents in the electrolyte don’t get along with other parts of the battery,” said Arumugam Manthiram, a professor in the Cockrell School of Engineering’s Walker Department of Mechanical Engineering and the lead researcher on the project. “We have reduced that risk from the equation to create a safer, more stable battery.”
In addition to the safety improvement, this new, sodium-based battery represents a less expensive alternative to the lithium-ion batteries that power smartphones, laptops, electric cars and more.
The battery also boasts strong performance. How long a battery lasts on a single charge tends to decline over time. The new sodium battery retained 80% of its capacity over 500 cycles, matching the standard of lithium-ion batteries in smartphones.
“Here we show a sodium battery that is safe and inexpensive to produce, without losing out on performance,” Manthiram said. “It is critical to develop alternatives to lithium-ion batteries that are not just on par with them, but better.”
Though the researchers applied this technique to a sodium battery, they said it could also translate to lithium-ion-based cells, albeit with different materials.
Lithium mining is expensive and has been criticized for its environmental impacts, including heavy groundwater use, soil and water pollution and carbon emissions. By comparison, sodium is available in the ocean, is cheaper and is more environmentally friendly.
Lithium-ion batteries typically also use cobalt, which is expensive and mined mostly in Africa’s Democratic Republic of the Congo, where it has significant impacts on human health and the environment. In 2020, Manthiram demonstrated a novel, cobalt-free lithium-ion battery.
This battery is also free of cobalt, as well as lithium. The other components are made of 40% iron, 30% manganese and 30% nickel.
Other authors on the paper are Jiarui He, Amruth Bhargav, Laisuo Su, Julia Lamb and Woochul Shin—all from the Cockrell School’s Materials Science and Engineering program and Texas Materials Institute—and John Okasinski of Argonne National Laboratory.