Metals are typically used as active materials for negative electrodes in batteries. Recently, redox-active organic molecules, such as quinone- and amine-based molecules, have been used as negative electrodes in rechargeable metal–air batteries with oxygen-reducing positive electrodes. Here, protons and hydroxide ions participate in the redox reactions. Such batteries exhibit high performance, close to the maximum capacity that is theoretically possible.
Furthermore, using redox-active organic molecules in rechargeable air batteries overcomes problems associated with metals, including the formation of structures called ‘dendrites,’ which impact battery performance, and have negative environmental impact. However, these batteries use liquid electrolytes—just like metal-based batteries—which pose major safety concerns like high electrical resistance, leaching effects, and flammability.
Now, in a recent study published in Angewandte Chemie International Edition, a group of Japanese researchers have developed an all-solid-state rechargeable air battery (SSAB) and investigated its capacity and durability. The study was led by Professor Kenji Miyatake from Waseda University and the University of Yamanashi, and co-authored by Professor Kenichi Oyaizu from Waseda University.
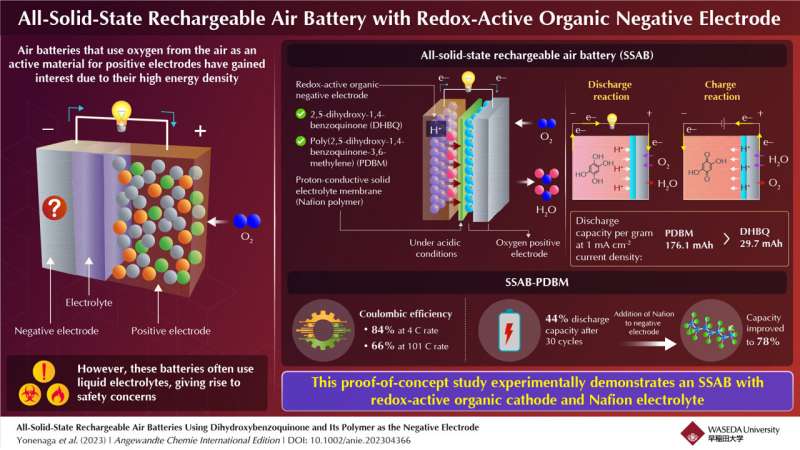
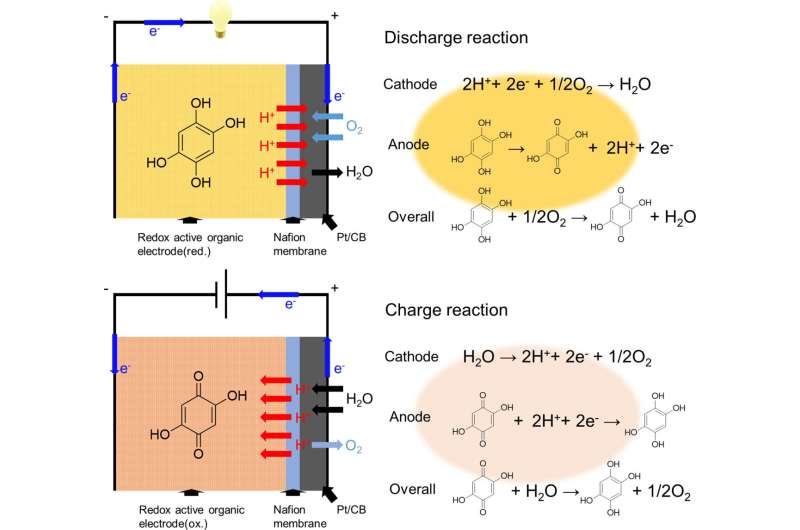
The researchers chose a chemical called 2,5-dihydroxy-1,4-benzoquinone (DHBQ) and its polymer poly(2,5-dihydroxy-1,4-benzoquinone-3,6-methylene) (PDBM) as active materials for the negative electrode due to their stable and reversible redox reactions in acidic conditions. In addition, they utilized a proton-conductive polymer called Nafion as the solid electrolyte, thereby replacing conventional liquid electrolytes.
“To the best of my knowledge, no air batteries based on organic electrodes and solid polymer electrolyte have been developed yet,” says Miyatake.
After the SSAB was in place, the researchers experimentally assessed its charge–discharge performance, rate characteristics, and cyclability. They found that unlike typical air batteries that use a metallic negative electrode and an organic liquid electrolyte, the SSAB did not deteriorate in the presence of water and oxygen.
Furthermore, replacing the redox-active molecule DHBQ with its polymeric counterpart PDBM formed a better negative electrode. While the per gram-discharge capacity of the SSAB-DHBQ was 29.7 mAh, the corresponding value of the SSAB-PDBM was 176.1 mAh, at a constant current density of 1 mAcm-2.
The researchers also found that the coulombic efficiency of SSAB-PDBM was 84% at 4 C rate, which gradually decreased to 66% at 101°C rate. While the discharge capacity of SSAB-PDBM reduced to 44% after 30 cycles, by increasing the proton-conductive polymer content of the negative electrode, the researchers could significantly improve it to 78%. Electron microscopic images confirmed that the addition of Nafion improved the performance and durability of the PDBM-based electrode.
This study demonstrates the successful operation of an SSAB comprising redox-active organic molecules as the negative electrode, a proton-conductive polymer as the solid electrolyte, and an oxygen-reducing, diffusion type positive electrode. The researchers hope that it will pave the way for further advancements. “This technology can extend the battery life of small electronic gadgets such as smartphones and eventually contribute to realizing a carbon-free society,” concludes Miyatake.