Water blankets around 70 percent of the Earth’s surface. Moreover, 97 percent of all the water on earth is seawater, which is undrinkable because of its salt content. But what if we could harness its potential as a new source of renewable energy?
Recently, a research team led by Professor Changshin Jo (Graduate Institute of Ferrous & Energy Materials Technology (GIFT), Department of Chemical Engineering) and Ph.D. candidate Hyebin Jeong (Chemical Engineering) at POSTECH has made strides in this area by confirming the superior performance of seawater batteries (SWBs) that incorporate chelating agents. Their findings were published in Chemical Engineering Journal.
Lithium-ion batteries have become ubiquitous in portable electronic devices and automotive batteries. However, they are not without limitations, as they present a risk of explosion and may become unusable if lithium supplies are depleted. To address these challenges, the development of next-generation batteries is currently underway.
Among them, seawater batteries represent a promising option that utilizes Na-ions found in seawater to generate energy. These batteries offer the distinct advantage of easy resource accessibility and are environmentally friendly, as they require no separate treatment processes.
The high salinity of seawater can be attributed to the presence of Na-ions, which are utilized by seawater batteries to generate and store electrical energy as they move back and forth between the cathode and anode.
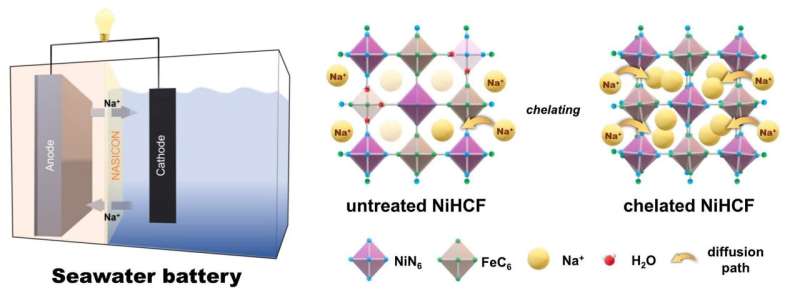
However, one of the challenges in suing nickel hexacyanoferrate (NiHCF) as an intercalation cathode material for SWBs is the high occurrence of defects during fabrication. To address this issue, the research team synthesize NiHCF with a chelating agent (Sample A) and compared its performance with untreated NiHCF (Sample B) to evaluate the effectiveness of the chelating agent.
A look at the two samples under a microscope reveals the striking difference in their shape and structure. Sample B consists of randomly aggregated nanosized primary particles to form micro-level particles, whereas Sample A comprises individual 200-300 nanometer-sized cubic-shaped particles. Although the individual particle size of Sample B is smaller, it is less advantageous for battery production due to the aggregation of multiple particles into larger cohesive structures.
The researchers additionally assessed the electrochemical performance of both samples. Firstly, they measured the water content, and it was found that Sample A had lower water content than Sample B did. Generally, higher water content tends to impede electrochemical performance. Furthermore, measurements of current and voltage showed that Sample A had high energy efficiency and capacity.
The research team achieved a groundbreaking feat by performing 2,000 cycles of charging and discharging on batteries using two samples, where Sample A demonstrated a remarkable capacity retention rate of approximately 92.8%. Furthermore, the defect generation rate, a previous drawback of NiHCF, was observed to decrease to 6% in Sample A.
The results of the study demonstrate the superior performance achieved by adding a chelating agent to nickel hexacyanoferrate and using this as a cathode material in seawater batteries. This discovery can promote the development of seawater batteries as a promising candidate for next-generation energy storage systems.