Skoltech researchers have found a way to produce hydrogen from natural gas with 45% efficiency right in the gas field by injecting steam and a catalyst into a well and adding oxygen to ignite the gas. Catalyst-assisted combustion produces a mixture of carbon monoxide and hydrogen, from which the latter can be easily extracted. This technology will help accelerate the transition from fossil fuels to clean hydrogen power. The study was published in Fuel.
Approximately 80% of energy comes from fossil fuels, such as oil and natural gas, which release carbon dioxide when burned, threatening the environment and contributing to climate change. Although gas is considered “cleaner” than oil with its many toxins, it still emits carbon dioxide when burned, making gas a threat to the environment.
Hydrogen, which emits nothing but water vapor, could be a healthier alternative. However, the widespread use of this “green” energy source is hampered by production difficulties.
For the first time, a team from the Skolkovo Institute of Science and Technology in Moscow has proposed extracting hydrogen from the reservoirs of natural gas fields, which are rich in hydrocarbons that contain a large amount of hydrogen at the molecular level. This means that hydrocarbons, once converted, can yield an abundance of “green” fuel.
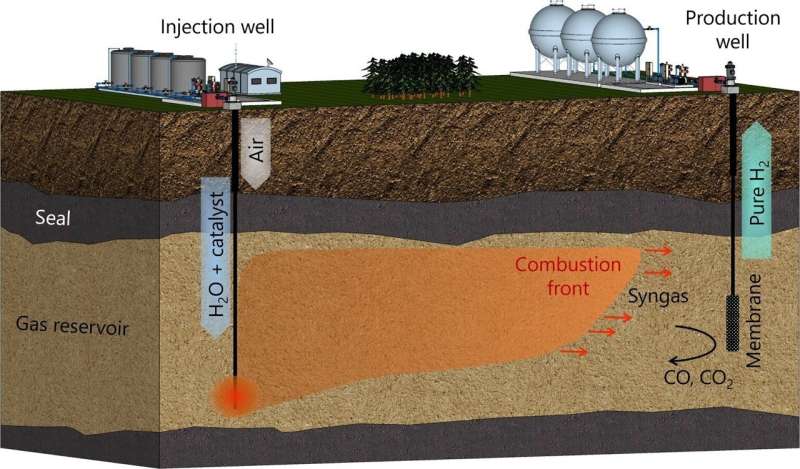
The team has proposed an efficient, multi-step process for producing hydrogen from gas fields. First, steam is injected into the well along with a catalyst that will later help separate hydrogen from the natural gas components. Then, air or pure oxygen is pumped in to ignite the gas directly in the reservoir.
Assisted by the steam and catalyst, the natural gas burns and is converted into a mixture of carbon monoxide and hydrogen. The carbon dioxide formed from the carbon monoxide remains in the reservoir and does not contribute to the greenhouse effect.
At the final stage, hydrogen is extracted from the well through a membrane that blocks other combustion products, leaving the carbon monoxide and carbon dioxide permanently trapped underground.
The researchers tested their process in lab reactors that simulated a real gas reservoir environment. They placed crushed rock in the reactor and then pumped in methane, the main component of natural gas, along with steam and a catalyst, and then oxygen. The pressure inside the reactor was maintained at a level typical of gas reservoirs (eighty times higher than atmospheric pressure).
As the experiment progressed, the team analyzed the composition of the gases in the reactor to assess the efficiency of the methane conversion into hydrogen. It turned out that most of the hydrogen −45% of the total gas volume—was formed at 800°C with large amounts of steam injected into the reactor.
To make the reaction as efficient as possible, there should be four times more steam than natural gas. The researchers chose the 800°C temperature because it is easily achieved in natural gas combustion and does not need to be artificially maintained.
The hydrogen yield also depended on the composition of the rock. For example, in experiments with porous alumina, the hydrogen yield reached 55%. The higher efficiency in this case is explained by the fact that alumina is inert, i.e. it does not react with the surrounding elements. Natural rock contains other, more active minerals that can react with the components of the gas mixture and affect the hydrogen yield.
“All the stages of the process are based on well-established technologies that have not previously been adapted for hydrogen production from real gas reservoirs. We have demonstrated that our approach can help convert hydrocarbons into ‘green’ fuels in the field environment with an efficiency of up to 45%. In the future, we plan to test our method in real gas fields,” says Elena Mukhina, Ph.D., a senior research scientist at Skoltech Petroleum and the leader of the project.