In recent years, engineers and material scientists have been trying to create increasingly advanced battery technologies that are charged faster, last longer, and can store more energy. These batteries will ultimately play a crucial role in the advancement of the electronics and energy sector, powering the wide range of portable devices on the market, as well as electric vehicles.
Lithium-ion batteries (LiBs) are currently the most widespread batteries worldwide, powering most electronics we use every day. Identifying scalable methods to increase the speed at which these batteries charge is thus one of the primary goals in the energy field, as it would not require switching to entirely new battery compositions.
Researchers at Huazhong University of Technology in China recently introduced a new strategy to develop fast-charging LiBs containing a graphite-based material. Their proposed battery design, outlined in a paper published in Nature Energy, was found to successfully speed up the charging time of LiBs, while also allowing them to retain much of their capacity even after they are charged thousands of times.
“Li+ desolvation in electrolytes and diffusion at the solid–electrolyte interphase (SEI) are two determining steps that restrict the fast charging of graphite-based lithium-ion batteries,” Shuibin Tu, Bao Zhang and their colleagues wrote in their paper.
“We show that the low-solvent-coordination Li+ solvation structure could be induced near the inner Helmholtz plane on inorganic species. Specifically, Li3P could enable a lower Li+ desolvation barrier and faster Li+ diffusion capability through the SEI in comparison to the regular SEI components.”
Essentially, the researchers carried out a series of tests to assess how various solid electrolyte interface (SEI) components affect the so-called Li+ solvation structure, which could in turn decrease the time required for a battery to charge. Ultimately, they identified a combination of materials that could improve the efficiency of the so-called Li+ desolvation process, enabling the rapid migration of Li+ ions across the SEI.
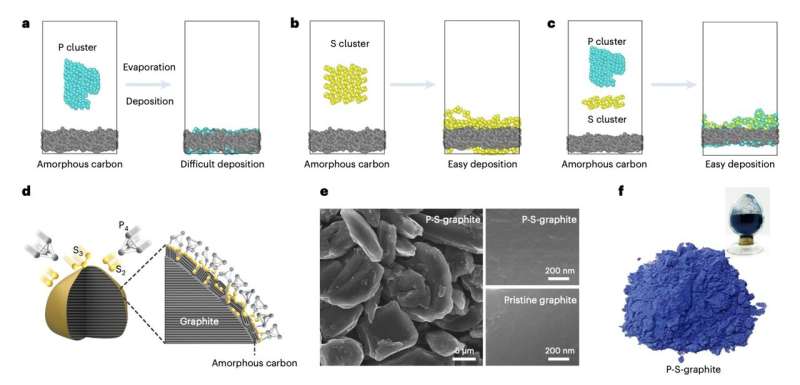
The team then identified a promising anode based on a material dubbed P-S graphite, which is comprised of an ultrathin layer of phosphorus on top of a graphite surface. They fabricated these anodes and integrated them in LiB cells, to then evaluate their performance experimentally.
“We construct an ultrathin S-bridged phosphorus layer on a graphite surface, which in situ converts to crystalline Li3P-based SEI with high ionic conductivity,” Tu, Zhang and their colleagues wrote. “Our pouch cells with such a graphite anode show 10 min and 6 min (6C and 10C) charging for 91.2% and 80% of the capacity, respectively, as well as 82.9% capacity retention for over 2,000 cycles at a 6C charging rate.”
Overall, the paper by Tu, Zhang and their colleagues highlights the key role of SEI components and structural considerations in influencing the speed at which LiB cells charge. In the future, their proposed approach and their promising experimental results could contribute to the development of increasingly fast-charging and durable LiBs, which could help to meet the pressing demands of the electronics industry.
“Our work highlights the importance of interfacial chemistry for the Li+ solvation structure and SEI formation and could serve as a guide for the design of effective SEI components for fast-charging LiBs,” the researchers conclude in their paper.