Skoltech researchers have used an inexpensive 3D-printing technique to demonstrate the manufacturing of a ceramic part of a highly complex shape, which could be used in fuel cells, a promising technology for efficient and environmentally friendly electrical power generation.
The intricate ceramic structure, which cannot be obtained by traditional manufacturing techniques, would enable fuel cells to produce more power so that more wasteful energy sources could be phased out sooner. The study is reported in the journal Ceramics International.
One of the alternatives to burning natural gas or other fossil fuels at power plants or in combustion engines is using solid-oxide fuel cells. SOFCs can generate electricity for industrial facilities or homes, including in remote off-grid locations, as well as for boats, cars, and even satellites. The chief advantages are efficiency and resilience, as well as sustainability, while high operation temperatures and the need for new advanced materials are the factors hindering their widespread adoption.
SOFCs generate electricity by consuming methane or other hydrocarbons on site, right where the power is needed, so they are a good solution for backup power and other systems sensitive to outages. The fuel-to-power conversion in SOFCs does not involve combustion, pushing their electrical efficiency to about 60%, compared with 45% for a simple-cycle gas-fired power plant. In both cases, some of the waste heat can be salvaged, boosting the efficiency, but the upshot is that per 1 cubic meter of natural gas consumed, a fuel cell will yield more electricity than a power station.
As for the environmental benefits, combustion-free gas oxidation in a fuel cell does not emit pollutants like nitrogen oxides and sulfur dioxide or harmful aerosol particles with the exhaust gas. Also, carbon emissions in general are 40%-50% lower for a solid-oxide fuel cell running on natural gas compared with the national power grid, as reported by German and U.S. SOFC manufacturers.
The principal components of an SOFC are a cathode, an anode, and a layer of ceramic electrolyte material between them, whose chief feature of merit is ionic conductivity. It determines how well the electrolyte conducts oxygen ions, facilitating the chemical reaction that yields useful energy. The higher this parameter is, the more power the SOFC provides. Ionic conductivity depends on the electrolyte material used, how this material is structured, and the temperature at which the fuel cell is operated.
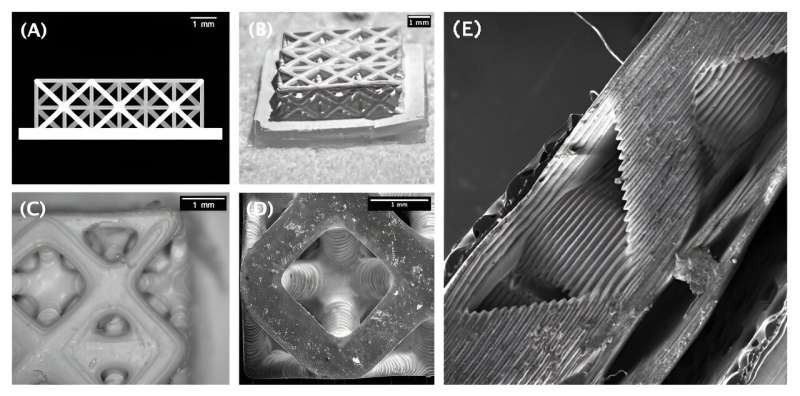
Skoltech researchers addressed the structure aspect, putting two common ceramic electrolyte materials into a complex shape called a hierarchical lattice structure. Complex structure maximizes the ionic conductivity, but is unattainable without advanced 3D printing technology. The materials used by the team are known as scandia-stabilized zirconia and yttria-stabilized zirconia. The former are suitable for SOFCs operating at 1,000 degrees Celsius, the latter at about 750 C. Both were fed into a 3D printer, which relies on the microstereolithography technology—and a movie projector, of all things.
The team assembled a low-cost demonstration system for high-precision 3D printing. As the ceramic part is being manufactured, it must be exposed to ultraviolet light to cure (harden) the polymer binder that holds the material together, resulting in a semifinished product, technically known as the 3D green part. The light has to be delivered with much precision if a complex structure is desired. To achieve this in their demonstration printer, the researchers used an office projector based on the digital light processing technology—a device often used for office presentations and the like.
After 3D printing, the UV-cured polymer binder is burned out of the semifinished part in the furnace. At the final stage, the porous ceramic sample is sintered to remove residual porosity and give the ceramics its strength. The study’s lead author, Skoltech MSc alumnus Igor Pchelintsev, suggested an innovative solution that amounts to combining the two steps—burning and sintering—in a single process. The research team developed, described, and carried out the 3D printing procedure, including optimizing the printing paste composition, post-processing the manufactured items, and testing their electrical properties.
“We have shown that 3D printing, and the inexpensive microstereolithography setup in particular, can actually produce complex structures from one experimental and one commercial-grade material used in emerging solid-oxide fuel cells,” Pchelintsev summed up. “This is a step toward improving the efficiency of fuel cells to the point where they could compete with and phase out dirtier energy sources.”
Now that the properties of the materials have been optimized in a laboratory setting, the next step would be to create one-off prototypes of actual solid-oxide fuel cells incorporating the 3D-printed lattice structures.