Researchers at the University of Colorado have developed a new and efficient way to produce green hydrogen or green syngas, a precursor to liquid fuels. The findings could open the door for more sustainable energy use in industries like transportation, steelmaking and ammonia production.
The new study, published Aug. 16 in the journal Joule, focuses on the production of hydrogen or syngas, a mixture of hydrogen and carbon monoxide that can be converted into fuels like gasoline, diesel and kerosene. The CU Boulder team lays the groundwork for what could be the first commercially viable method for producing this fuel, entirely using solar energy. That might help engineers to generate syngas in a more sustainable way.
The group was led by Al Weimer, professor in the Department of Chemical and Biological Engineering.
“The way I like to think about it is some day when you go to the pump you’ll have, for example, unleaded, super unleaded and ethanol options, and then an additional option being solar fuel, where the fuel is derived from sunlight, water and carbon dioxide,” said Kent Warren, one of two lead authors of the new study and a research associate in Chemical and Biological Engineering. “Our hope is that it will be cost-competitive to the fuels sourced from the ground.”
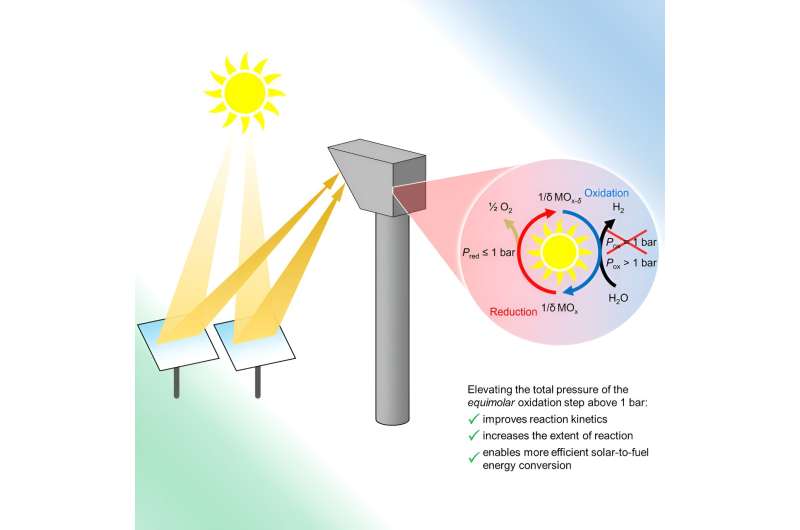
Traditionally, engineers produce hydrogen gas through electrolysis, or using electricity to split molecules of water into hydrogen and oxygen gas. The team’s “thermochemical” approach, in contrast, uses heat generated by solar rays to complete those same chemical reactions. The methods can also split molecules of carbon dioxide pulled from the atmosphere to produce carbon monoxide.
Scientists had previously shown that such an approach to making hydrogen and carbon monoxide was possible, but might not be efficient enough to produce syngas in a commercially viable manner.
In the new study, the researchers demonstrated that they can conduct these reactions at elevated pressures, in part by employing iron-aluminate materials, which are relatively inexpensive and abundant in the Earth. Those higher pressures allowed the team to more than double its production of hydrogen.