Tremendous interest in high-security and high-energy batteries in energy-storage systems has led to the rapid development of all-solid-state lithium batteries (ASSLBs). However, the electrochemical performance of ASSLBs is still inferior to that of liquid batteries due to the high ion transport resistance at the solid-state electrode/electrolyte interface. When the goal of high energy density is achieved by increasing the loading of electrodes, fast ion transport within electrodes remains an important challenge for solid-state batteries.
In a new research article published in the National Science Review, an energy technology research team led by Professor Jiajun Wang from Harbin Institute of Technology (HIT) presents results that reveal that the degradation of the electrochemical performance of solid-state electrodes originated from the imbalance of ion transport and electrode reaction.
A tortuosity-gradient thick electrode strategy (20 mg cm-2) was further proposed, which promotes fast charge transport, mitigates the heterogeneous solid-state reaction, enhances electrochemical activity and extends cycle life in thick solid-state electrodes.
First, the team investigated the effect of electrode thickness on the electrochemical performance of solid-state batteries through advanced synchrotron radiation analysis methods. Unlike liquid electrolytes in liquid-electrolyte batteries that can easily be infiltrated through the interface and porous electrodes, lithium ions can only transport slowly across the solid–solid interfaces in ASSLBs.
When the longitudinal distance transport path within the solid-state electrode increases, lithium ions cannot be smoothly transported between the solid-state electrolyte and the current collector, resulting in reaction heterogeneity and concentration gradients of lithium ions along the longitudinal axis of the thick electrode, eventually leading to battery failure.
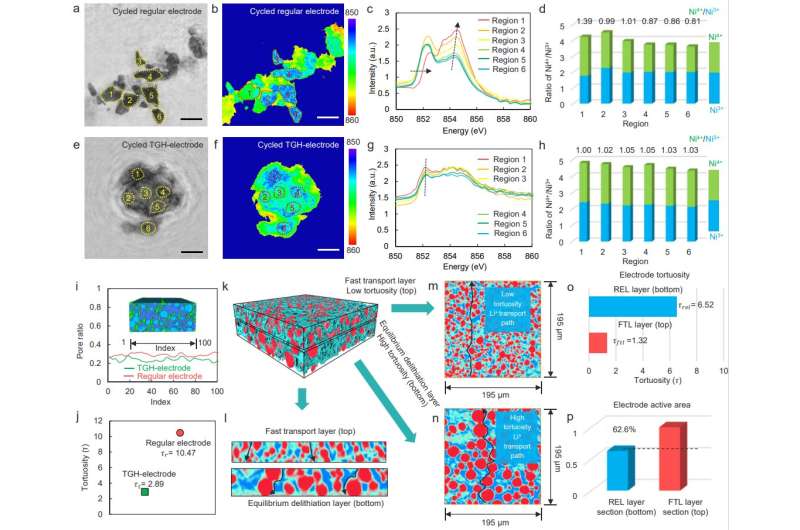
Further, the team fabricated a tortuosity-gradient solid-state electrode (TGH-electrode), which recasts the Li+ transport path in the solid-state electrode and achieves a balance between ion transport and reaction rates. The TGH-electrode composed of a fast transport layer (FTL) with an ionic percolation network and reaction equilibrium layer (REL) with large-sized particles. Coupling synchrotron radiation tomography, machine learning and electrochemical test analysis shows that the TGH-electrode can suppress the performance degradation of solid-state thick electrodes.
Finally, the team used advanced synchrotron radiation ptychography and tomography to analyze the morphological evolution and Ni chemical state distribution of secondary particles in the TGH-electrode with the advantages of high resolution and 3D imaging. Owing to the lower tortuosity provided by small particles in the FTL, the FTL has a short vertical ion-transport path, ensuring that Li+ can be transported rapidly from the electrolyte side to the current collector side.
The REL layer has a small specific surface area and only a low Li+ flux is required to achieve a specific delithiation level, which provides the possibility of equilibrium between Li+ transport and delithiation in the TGH-electrode. The synergy between the FTL and the REL maintains the equilibrium between ion transport and delithiation in the TGH-electrode, improving the cathode utilization and promoting the cycle stability of ASSLBs.