In a new Nature Energy study, engineers report progress toward lithium-metal batteries that charge quickly—as fast as an hour. This fast charging is thanks to lithium metal crystals that can be seeded and grown—quickly and uniformly—on a surprising surface. The trick is to use a crystal growing surface that lithium officially doesn’t “like.” From these seed crystals grow dense layers of uniform lithium metal. Uniform layers of lithium metal are of great interest to battery researchers because they lack battery-performance-degrading spikes called dendrites. The formation of these dendrites in battery anodes is a longstanding roadblock to fast-charging ultra-energy-dense lithium-metal batteries.
This new approach, led by University of California San Diego engineers, enables charging of lithium-metal batteries in about an hour, a speed that is competitive against today’s lithium-ion batteries. The UC San Diego engineers, in collaboration with UC Irvine imaging researchers, published this advance aimed at developing fast-charging lithium-metal batteries on Feb. 9, 2023, in Nature Energy.
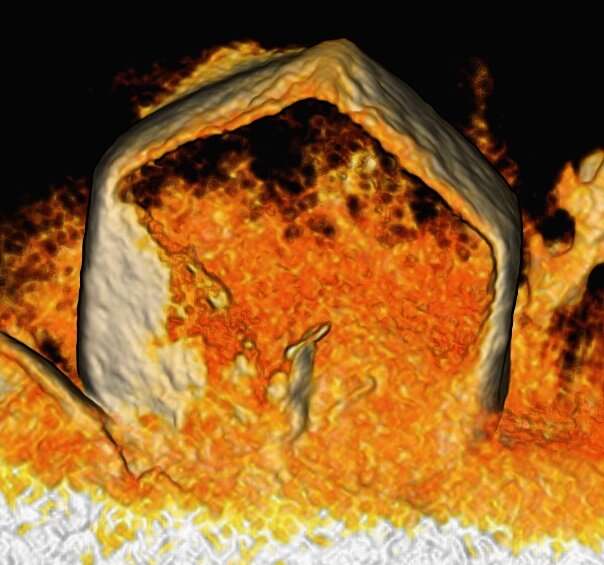
To grow lithium metal crystals, the researchers replaced the ubiquitous copper surfaces on the negative side (the anode) of lithium-metal batteries with a lithiophobic nanocomposite surface made of lithium fluoride (LiF) and iron (Fe). Using this lithiophobic surface for lithium deposition, lithium crystal seeds formed, and from these seeds grew dense lithium layers—even at high charging rates. The result was long-cycle-life lithium-metal batteries that can be charged quickly.
“The special nanocomposite surface is the discovery,” said UC San Diego nanoengineering professor Ping Liu, the senior author on the new paper. “We challenged the traditional notion of what kind of surface is needed to grow lithium crystals. The prevailing wisdom is that lithium grows better on surfaces that it likes, surfaces that are lithiophilic. In this work, we show that is not always true. The substrate we use does not like lithium. However, it provides abundant nucleation sites along with fast surface lithium movement. These two factors lead to the growth of these beautiful crystals. This is a nice example of a scientific insight solving a technical problem.”
The new advance led by UC San Diego nanoengineers could eliminate a significant roadblock that is holding back widespread use of energy-dense lithium-metal batteries for applications like electric vehicles (EVs) and portable electronics. While lithium-metal batteries hold great potential for EVs and portable electronics because of their high charge density, today’s lithium-metal batteries must be charged extremely slowly in order to maintain battery performance and avoid safety problems.
The slow charging is necessary to minimize the formation of battery-performance-wrecking lithium dendrites that form as lithium ions join with electrons to form lithium crystals on the anode side of the battery. Lithium crystals build up as the battery charges, and the lithium crystals dissolve as the battery discharges.