A new publication in Opto-Electronic Advances from researchers including Wei Li of the Chinese Academy of Sciences discusses pathways toward a more sustainable electrocatalyst for efficient hydrogen technology.
When fossil fuels are burned, massive amounts of carbon dioxide, a greenhouse gas, are released into the atmosphere. Greenhouse gases, which trap heat in Earth’s atmosphere, are to blame for global warming. Sea level rise, harsh weather, biodiversity loss, species extinction, food scarcity, worsening health, and increased poverty are all risks associated with global average warming of above 1.5 degrees Celsius.
Slowing global warming is one of the most pressing challenges confronting humanity today. A critical part of addressing such climate changes is reducing the use of fossil fuels and shifting toward renewable energy sources with zero or negative carbon emissions. The good news is that numerous nations are already making efforts to address this issue. For example, many countries have established challenging goals for reducing their reliance on fossil fuels and converting to renewable energy. Solar, geothermal, hydroelectric, wind and biomass are examples of renewable energy sources that can produce energy without accelerating global warming.
With the world in search of a suitable substitute for fossil fuels, researchers realized that hydrogen may be an effective energy carrier and could be a good substitute for fossil fuels. But there was an issue: finding hydrogen in a free state is impossible. As it is a highly reactive non-metal, it never exists freely in nature and is only produced from other sources of energy.
Then it was discovered that a method called electrochemical oxidation/reduction of radicals at electrodes, which is environmentally friendly and sustainable, could assist with the production of hydrogen, ammonia, hydrocarbons, and other fuels. Electrochemical fuel generation for hydrogen and oxygen is mostly carried out through hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), respectively.
But this hydrogen technology requires highly active and stable catalysts for the HER, and platinum (Pt), a rare element, is used in water-splitting devices. The platinum catalyst, makes this technology much more expensive, and platinum production involves toxic chemicals that affect our ecosystem.
Moreover, the non-local concentration of radicals around the platinum electrocatalyst greatly slows the speed of reaction. Speeding up this reaction is possible by bringing up the radical concentration via large electrode, potentially with the help of electricity, this solution is still expensive.
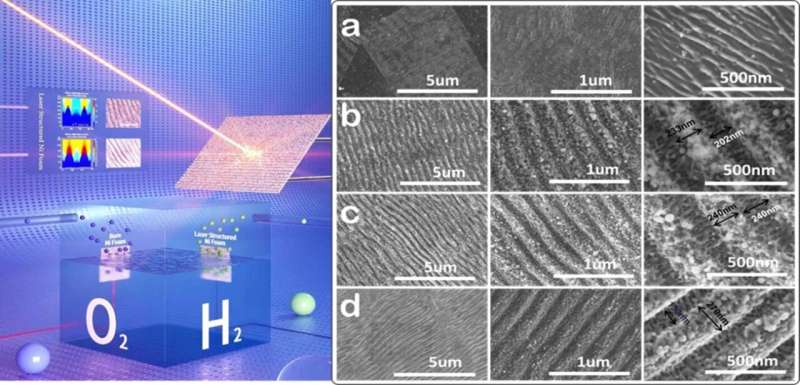
The authors of the study have developed a physical and versatile design approach to boost the electrocatalytic fuel generation performance to a wide range through their high-performance electrodes (LIPSS). They say, “The hierarchical LIPSSs on electrodes with periodic ridges and grooves of 100-300 nm width must be covered with spherical nanoparticles (NPs) sized 3-94 nm diameters and then the localized electric field-induced enhancement in the reagent concentration effect at these periodic ridges and NPs could considerably enhance the performance of electrochemical fuel generation of HER and OER.”
In experiments to test the performance of this catalyst, the authors discovered that by using this optimized morphology of the LIPSS pattern, the current electrode achieves the highest hydrogen generation rate of about 3×1016 molecules cm-2s-1 at a current density of 10 mA/cm2.
This value is achieved with ~45% less electrode potential than that of the Ni foam electrode without any LIPSS pattern. The precise and controlled fabrication of LIPSS on electrodes could significantly improve their performance as a sustainable electrocatalyst for efficient hydrogen generation. On the LIPSS patterned Ni foam substrate, the HER model electrocatalyst demonstrated 130 mV (40%) of lower 10 overpotentials in the HER and high stability. Furthermore, the OER model electrocatalyst on the LIPSS patterned NF substrate required 100 mV (25%) more 10 overpotentials in the OER with improved stability.
Additionally, when two LIPSS patterned electrodes were assembled simultaneously as anode and cathode in a cell, a low electric potential of 330 mV was enough to drive 10 mA/cm2 in the overall water splitting figure, compared to a similar cell made of pristine Ni foam electrodes. The patterned LIPSS electrocatalysts operate at significantly lower electrical potentials, demonstrating that the femtosecond laser patterning approach has a high likelihood of producing green catalysts.
Based on the above process, it is believed that the new insights presented in this study would pave the way for the demonstration of a single-step, fast, and better physical approach to electrode surface patterning that can be applied to any metal and semiconductor catalysts to reduce the required electrical power in various electrochemical reactions.
According to recent findings from the Chinese Academy of Sciences (CAS), Li and his research group are getting closer to achieving this objective. Their technique is still in the research stage but appears to be a promising source of power. Creating a so-called hydrogen-extracting catalyst that is reliable and sustainable for Earth is their ultimate ambition. The authors of the article look forward to seeing their method on the market in the next few years, and to see its impact grow around the world.