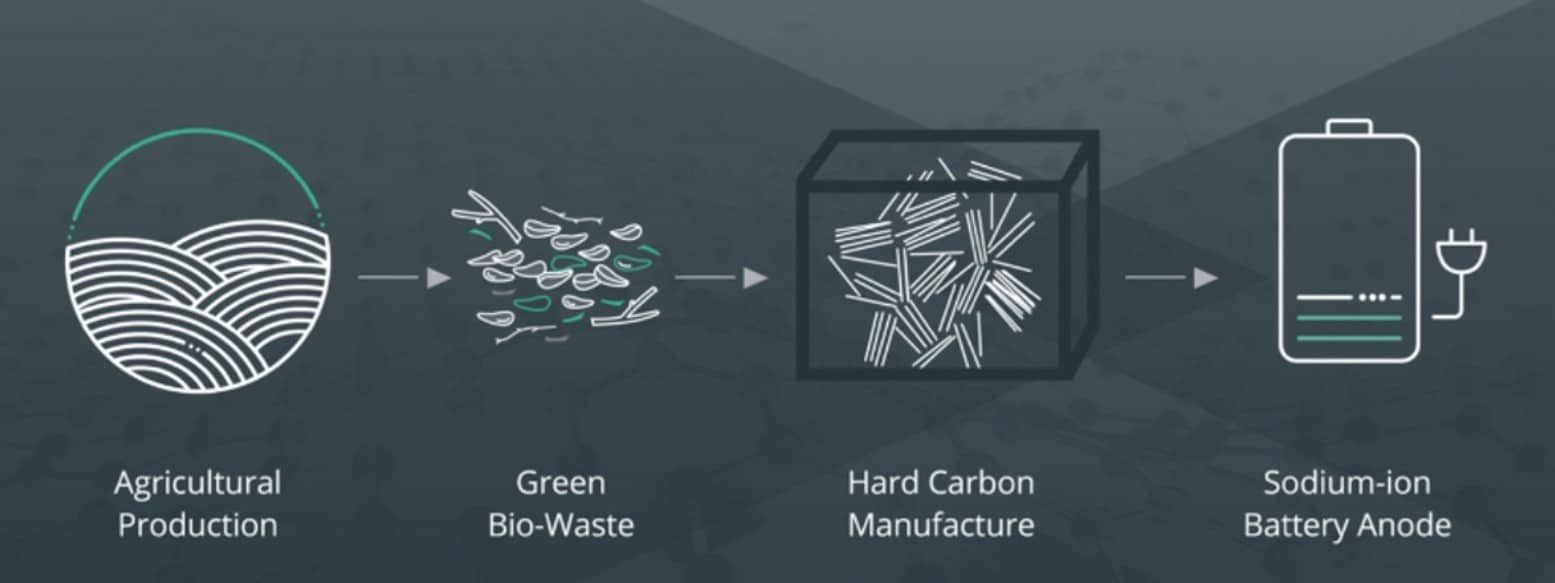
How might bio waste materials eventually be incorporated into sodium-ion batteries? That’s the question that a new strategic partnership hopes to answer. Australia-based Sparc Technologies will collaborate with the Queensland University of Technology (QUT) with the hope of identifying a significant alternative to lithium-ion batteries. Sustainable battery materials are in demand with the mass consumption of technology devices and electric vehicles, and sodium ion batteries (NIBs) have attracted worldwide attention for next generation energy storage systems.
A high performing, less expensive, sustainably sourced anode material for sodium-ion batteries could meet the need for what is a growing alternative battery technology. Researchers believe that sodium-ion batteries have significant potential for grid scale storage and mobile application. Wood Mackenzie expects sodium-ion batteries to take a share in passenger EVs and energy storage, reaching 20 GWh by 2030 in its base-case scenario.
Existing hard carbon materials are typically sourced from carbonaceous precursors such as pitch — a by-product of the oil and gas industry — which undergo lengthy heating at high temperatures. This is a very energy consuming process, which, combined with a high emission feed stock, has significant environmental impacts. The Sparc/ QUT project will develop a novel process for the production of hard carbon using low cost sustainably sourced green bio waste targeting the sodium-ion battery industry. The hard carbon materials will be characterized and tested in a sodium-ion cell format at QUT’s facilities for battery development and testing, including the National Battery Testing Center and Central Analytical Research Facility.
Advantages of sodium-ion batteries versus lithium-ion batteries, as outlined in a joint Sparc/QUT press release, are:
- Lower cost and greater availability of raw materials
- Safety and ease of transport
- Similar manufacturing techniques to lithium-ion and, therefore, can use the same production facilities
The Sustainable Hard Carbon Anode project complements Sparc’s existing businesses in graphene and renewable energy. Graphene is a two-dimensional material made of carbon atoms arranged in a hexagonal lattice; this structure creates unique and powerful properties that can be imparted on products to improve performance.
The framework for long term cooperation allows Sparc and QUT to work together to identify and undertake new projects. Sparc managing director Mike Bartels commented, “Sparc is excited to join with QUT in a Strategic Partnership, commencing with a project in the battery anode space with the development of a novel process for the production of hard carbon. Using readily available, sustainable bio waste material will provide Sparc with a strong environmental value proposition when compared with conventional sources of hard carbon.”
Bartels added that the materials used in sodium-ion batteries are accessible, not challenged in supply as is the case with lithium-ion batteries, and offer enhanced safety for industrial scale energy storage.
What’s the Story with Sodium-Ion Batteries?
Sodium has many attributes as a candidate for new battery technology, largely because it is inexpensive and abundant. Then again, its limited performance has stymied many other research projects and the hope for large scale applications.
Indeed, the severe instability of the solid electrolyte interphase (SEI) formed during repeated cycling hinders the development of NIBs. One of the dilemmas is to stabilize the liquid core of the battery to prevent performance issues of past sodium-ion battery projects. As a battery goes through repeated cycles of charging and discharging, it loses its ability to hold a charge.
Cornell University researchers have also uncovered the source of a persistent problem limiting the durability of sodium-ion batteries. The poor durability stems from a specific atomic reshuffling in the battery’s operation – the P2-O2 phase transition – as ions traveling through the battery disorder crystal structures and eventually break them. While the phase transition has been of interest to researchers, the mechanisms behind it have been difficult to study, especially during battery operation.
What will it take for sodium-ion Sparc/QUT R&D to produce similar reliability results as the current leading battery sources?
The Contest Among Battery Technologies
The 3 battery technologies currently widely utilized are lead, lithium, and vanadium redox flow. There are a number of factors to consider when selecting the most appropriate battery chemistry to meet a company’s energy storage needs.
Maturity of technology: Lead is the most commercially mature of the three battery technologies and has been the primary energy storage solution for many years. Lithium is another commercially mature technology in the scale necessary at this time. With its high energy density, lithium is currently the dominant battery technology for energy storage and comes in a wide variety of chemistry combinations. Vanadium redox flow battery technology has been around for over 50 years, but is the least commercially mature of the 3 chemistries.
Sustainability: Lead is the most sustainable of the 3 battery chemistries with a 99% recycle rate and a well-developed circular economy that reuses and recycles the lead, electrolyte and plastic components of used batteries. Vanadium is almost infinitely reusable, as the electrolyte that constitutes the bulk of a vanadium battery system can be dried out, purified as necessary, and then used in another system. Lithium is the least sustainable, with a recycling rate of less 5%, due to the cost and complexity of the process — a lithium battery must be dismantled and shredded, then melted down or dissolved in acid.
Time of operation: Vanadium is best suited for long duration energy storage (6 hours or more operating time). It has a larger footprint, but it is easier to expand. Lithium is appropriate for short to medium duration (from a few minutes to four hours operating time). Lead also works best for short to medium duration, especially in situations where depth of discharge is fairly shallow and low up front cost is a major gating factor.
Useful life: The useful life of a lithium battery is about 10 to 15 years, while vanadium can last more than 30 years. Lead batteries can have a useful life up to 30 years, depending on the design and applications.
Safety: All 3 battery systems are generally safe, assuming there are no defects or damage.
Supply chain: Lead is readily available and domestically produced. Domestic recycling provides 73% of the domestic demand for lead. The US has about 4% of lithium reserves, and it produces less than 2% of the world’s supply. Currently, there is no domestic US production of vanadium, leaving the US dependent on foreign sources.
Final Thoughts
Energy and climate concerns have increased the need for research towards electrical energy storage. Because of the unique advantages of sodium ion batteries, such as cost and nearly unlimited resources, the interest in them has increased drastically over the last several years. The Sparc/QUT project gives hope for additional resources of sustainable battery materials and the potential to incorporate more environmentally friendly and even less expensive options than lithium-ion batteries provide.