After an Astounding Surge in Patient Response Rate in their GOBLET Study and Earning an FDA Fast Track Designation, Wall Street Analysts are Touting Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) as a Universal STRONG BUY Rating, and Eyeing a Potential $15.00 Price Target (Currently at $2). Earlier This Year They Received an Immense Honor at the 2023 ASCO Annual Meeting—an Oral Presentation Slot. Don’t Miss this Chance to Explore this Rising Star in Cancer Research. Get Ahead of this News TODAY!
As we launched into 2023, a resounding echo from the past was reignited by President Joe Biden: The “Cancer Moonshot” initiative[1].
The Cancer Moonshot is a bold ambition aimed to halve the cancer rate within a quarter of a century, turning science fiction into reality and improving the lives of cancer survivors[2].
In response, US Congress was asked to fork over a whopping $2.8 billion to fund this extraordinary venture[3]. The Moonshot, like its astronomical namesake, stands as a beacon of hope and a challenge that has galvanized the biotech space.
Large pharmaceutical companies aren’t staying idle. Pfizer, as an example, put a massive $43 billion into acquiring Seagen, signaling a big move towards developing drugs to fight cancer[4]. That wasn’t the only multi-billion-dollar cancer acquisition so far this year, as TPG and AmerisourceBergen Corporation acquired OneOncology for $2.1 billion[5].
There were many big deals and company mergers in the biotech field in 2022, and we can expect even more exciting things in 2023 and beyond[6]. Interestingly, analysts at RBC Capital Markets, are pointing towards smaller, agile firms that are quickly gaining ground, stating “Small is the New Big” when it comes to the biotech sector[7].
So this Cancer Moonshot endeavor hasn’t just inspired hope; it’s also sparked a whole lot of activity in the biotech world.
Now, some of those sparks are becoming fires, and Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is one of them.
Just months after earning the FDA’s Fast Track Designation for its flagship drug[8], pelareorep, for treating advanced/metastatic pancreatic cancer, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) took center stage at the ASCO 2023 Annual Meeting, with a coveted oral presentation slot awarded by ASCO’s selection committee.
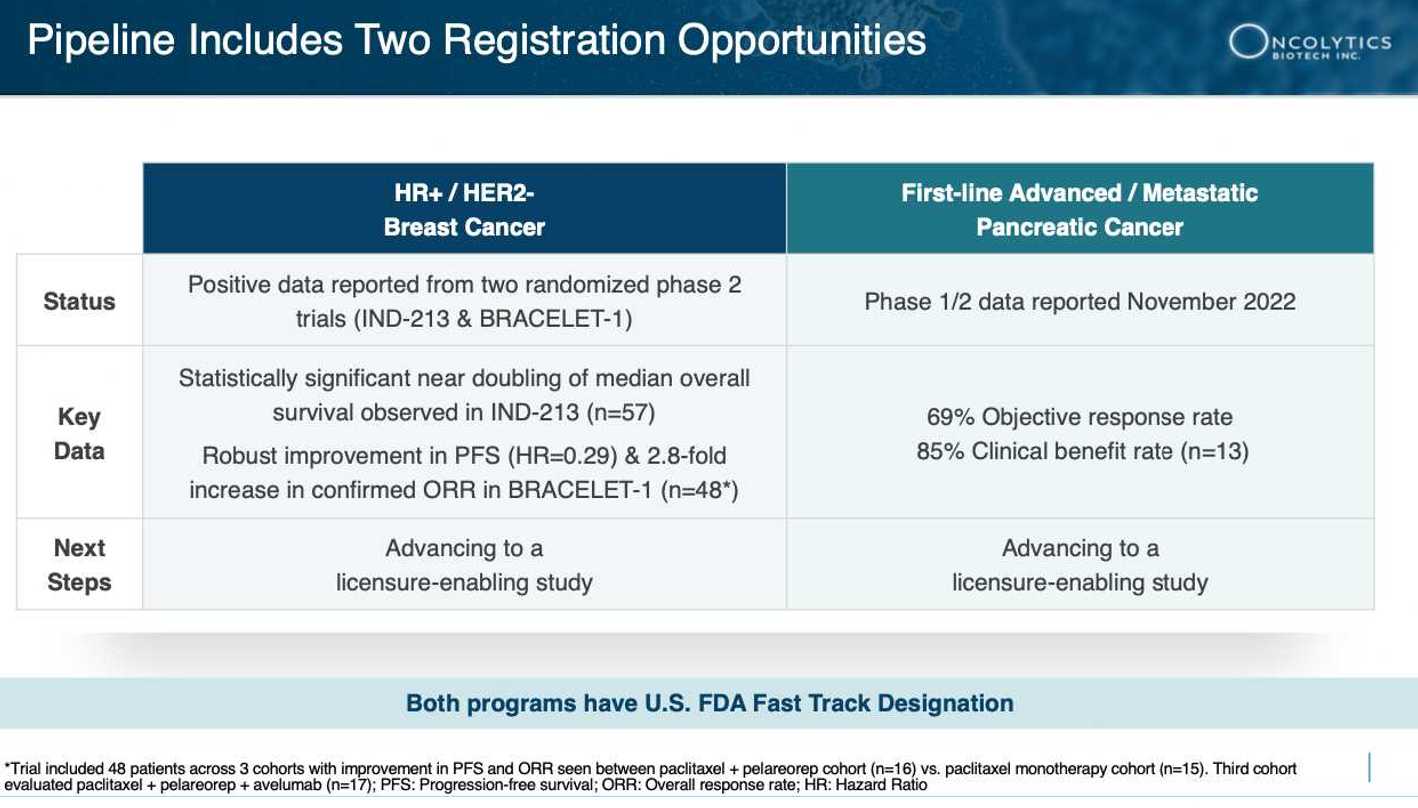
On top of this, data from Oncolytics’ BRACELET-1 metastatic breast cancer trial has already shown pelareorep’s prowess. Notable achievements include a reduced risk of disease progression by 71% (hazard ratio of 0.29) compared to paclitaxel monotherapy, 37.5% confirmed overall response rate (ORR) and a significant increase in 12-month progression-free survival to 32.8% for pelareorep-paclitaxel compared to 0% for paclitaxel monotherapy[9].
This paints a promising picture of Oncolytics’ potential, echoing the broader industry sentiment that smaller biotech firms might just be the David to the Goliaths of cancer treatment.
With promising drug trials, impressive presentations on the horizon, and an industry ripe for their advancements, let’s now take a deeper look at Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC)—a small company ready to take a giant leap in the fight against cancer.
8 Reasons Why Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) and Pelareorep Could Be Big News in Cancer Treatment
1 Top-Notch Collaborations: Pelareorep is attracting big names in the business. They’re teaming up with well-known companies like Pfizer, Merck KGaA, Roche, and Adlai Nortye. They’re also working with Merck & Co., Bristol-Myers Squibb, and Incyte.
2 Better Response Rate: Pelareorep’s response rate in pancreatic cancer is nearly 3X better than existing treatments[10]. This means it helped more patients fight off their cancer more effectively.
3 Complete Response Recorded: A complete response is when the cancer disappears entirely. This is a big deal. It happened to 1 out of 13 pancreatic cancer patients in the GOBLET study treated with pelareorep. That’s a MUCH higher rate than a previous trial[11] that had just one complete response out of 861 patients.
4 Fast Track Stamp: The FDA has given pelareorep a ‘Fast Track’ designation. This means the treatment could be available sooner to those who need it most. The FDA is fast-tracking pelareorep’s use with another cancer drug called atezolizumab and chemotherapy for advanced pancreatic cancer.
5 Boosting Other Treatments: Pelareorep can be used with immune checkpoint inhibitors (ICI), a type of drug that can be effective in fighting cancer, but only works for some people. Pelareorep might help more patients benefit from these inhibitors. The ICI market is expected to exceed $168 billion by 2031[12], despite as few as 1 in 5 patients responding to ICI therapy[13].
6 Exciting Data on the Horizon: Oncolytics Biotech has already shared promising data about pelareorep, and there’s more to come. They are expected to deliver new information from their colorectal cancer and pancreatic cancer cohorts of the GOBLET trial in the second half of 2023, which many believe could lead to further advances. As well, pelareorep was chosen for inclusion in PanCAN’s Precision PromiseSM pivotal phase 3 platform trial, which is expected to open in early 2024.
7 Experienced Team: The team behind pelareorep knows what they’re doing. They have over 150 years of combined experience in drug development and the biopharmaceutical industry.
8 Well-Funded: Oncolytics Biotech is in a good financial position. They have enough money to cover their key projects for the next 12 months and beyond.
The Experts’ Verdict: A Strong Buy
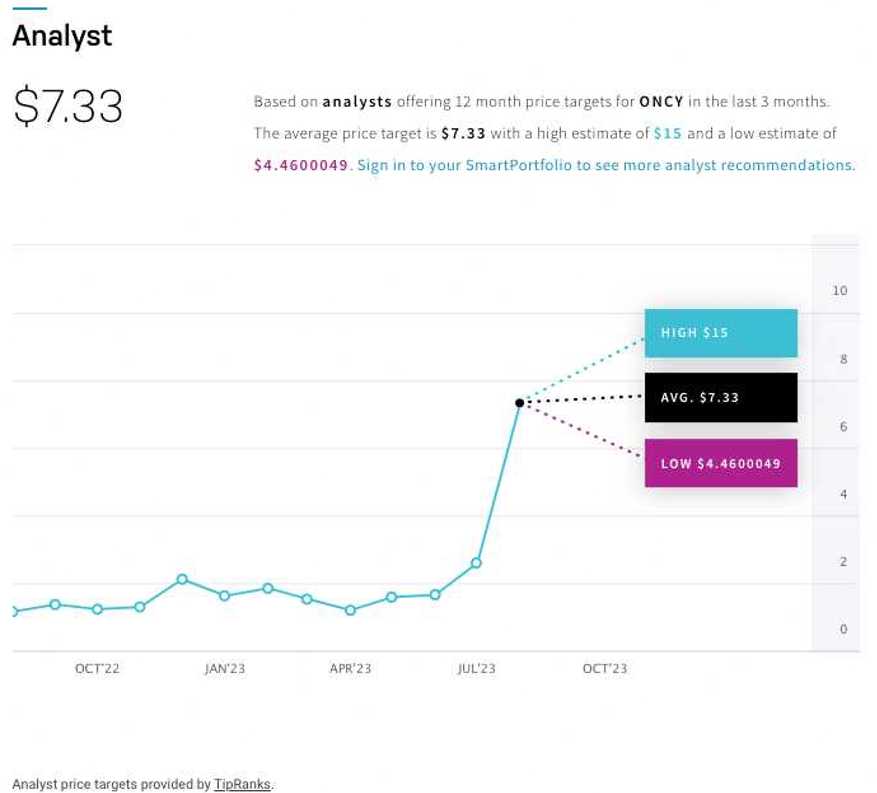
According to the consensus of analyst ratings on NASDAQ.com, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is receiving a STRONG BUY rating from experts. With an average price target of $7.33 and a high estimate of $15, they’re expecting a significant jump from its current share price of ~$2 as of August 15, 2023.
That’s a potential-high projected growth of over 6.5x its current price, or an increase of 650%!

So, what are these analysts seeing that the rest of the market might be missing?
While we can’t speak for them, we believe it should be crystal clear by now why ONCY is one of the most promising opportunities we’ve spotted in years.
You’ve already seen why Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is a star in the making, what’s coming down the pipeline very soon, and why NOW is the ideal time for investors to take action.
Accruing Accolades
Every year, ASCO draws companies from across the globe to showcase their latest cancer-fighting developments, with stakes running high. An impressive presentation at ASCO can be a game-changer for a company and could send its stock price rocketing upwards.
Just look at Kite Pharma’s journey. After an influential presentation at the ASCO meeting on June 6, 2016[14], their stock price started to climb from $53.72 and reached a high of $179.79 (a 234% gain) [15] within just over a year[16], culminating in an $11.9 billion takeover by Gilead Sciences (NASDAQ:GILD).
In 2022, Protagonist Therapeutics, Inc. (NASDAQ:PTGX) shared their developments at ASCO on June 7, and saw their stock price sprint from $8.81 to its current level of $24.45[17]— a surge of 177.5%.
Similarly, Arcellx, Inc. (NASDAQ:ACLX) presented clinical results at the ASCO meeting on June 5, 2022[18], which triggered a stock price rise from $13.30 to its current standing of $43.50—an impressive year-on-year increase of over 227%.
So at the ASCO 2023 Annual Meeting, it was Oncolytics Biotech Inc.’s (NASDAQ:ONCY) (TSX:ONC) turn to step up to the podium. They showcased their promising data from the BRACELET-1 metastatic breast cancer trial showing major improvements in response rates and progression-free survival with their treatment, pelareorep.
Could we be seeing the turning point Oncolytics Biotech has been waiting for?
NOTE: Oncolytics made their presentation on the morning of June 6, 2023, and then proceeded to go on a run from $1.55 on June 9th to a high of $3.24 by July 10—representing a jump of 109% in just over a month’s time!
There’s plenty more news to come as the year goes on, with data from the GOBLET study’s colorectal and pancreatic cancer cohorts expected later this year[19]. As well, pelareorep’s inclusion in PanCAN’s pivotal Phase 3 Precision PromiseSM platform trial in pancreatic cancer (more on this below) garnered even more attention—as the market swiftly rewarded ONCY with a US$15 million public offering shortly after.[20]
Pelareorep vs Pancreatic Cancer
 The Urgent Need for Better Pancreatic Cancer Treatments
The Urgent Need for Better Pancreatic Cancer Treatments
Pancreatic cancer is one of the deadliest forms of cancer, requiring urgent attention for effective treatments. Sadly, only about 12.5% of patients survive beyond five years after diagnosis, according to the U.S. National Cancer Institute[21]. Estimates suggest that by 2028, approximately 135,000 people in major markets will need treatment for advanced or metastatic pancreatic cancer[22].
Limited Efficacy of Existing Therapies
Current treatments, such as chemotherapy, show limited effectiveness. For example, when gemcitabine and nab-paclitaxel are used as the first line of defense against pancreatic cancer, they shrink the tumor in only about 25% of cases[23],[24],[25],[26]. Immune checkpoint inhibitors (ICIs) only work for less than 1% of patients[27], highlighting the desperate need for improved options.
Stunning Results from the GOBLET Study
The GOBLET study, a critical investigation into pelareorep’s effectiveness when combined with atezolizumab and chemotherapy, is painting an extraordinary picture. What’s been unveiled so far is nothing short of remarkable: an unprecedented 69% response rate in the initial batch of evaluated patients.
To fully understand the magnitude of this breakthrough, let’s put it in perspective. Compared to the usual 25% response rate observed with chemotherapy, this skyrockets way above the norm, nearly tripling the success rate. It’s not just about the numbers. It’s about the real, tangible hope this offers to patients who have been in dire need of more effective solutions.
Among the 13 evaluated patients, one experienced a complete response – a rare and exceptional outcome in the field of cancer treatment. A complete response means all signs of cancer disappeared with the treatment, an occurrence that’s almost unheard of in the context of this devastating disease.
Current treatments, such as chemotherapy, show limited effectiveness. For example, when gemcitabine and nab-paclitaxel are used as the first line of defense against pancreatic cancer, they shrink the tumor in only about 25% of cases. Immune checkpoint inhibitors (ICIs) only work for less than 1% of patients, highlighting the desperate need for improved options.
PanCAN’s Precision Promise Inclusion
These achievements have not gone unrecognized, as the Pancreatic Cancer Action Network (PanCAN) officially selected pelareorep for inclusion in its innovative adaptive Phase 3 clinical trial, Precision PromiseSM[28]. The Precision Promise study is designed to evaluate pelareorep in combination with a checkpoint inhibitor and the chemotherapeutic agents gemcitabine and nab-paclitaxel. This novel trial is expected to minimize the number of patients required to generate licensure-enabling data, accelerate development, reduce costs compared to a traditional trial, and support approval as a treatment for first-line metastatic pancreatic ductal adenocarcinoma.
Oncolytics expects to open the Precision Promise investigational treatment of pelareorep, checkpoint inhibitor, gemcitabine, and nab-paclitaxel in early 2024[29].
Pelareorep’s FDA Record
FDA Fast Track Designation: A Significant Achievement for Oncolytics Biotech
Recognizing the ground-breaking results from the GOBLET study, the FDA awarded Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) a Fast Track designation in December 2022[30]. This decision came on the heels of Oncolytics’ presentation at the Society for Immunotherapy of Cancer (SITC) 37th Annual Meeting, where they showcased the remarkable 69% objective response rate[31].
Fast Track designation is a significant milestone. It’s specifically designed to speed up the development and review of therapies that can treat severe conditions and address unmet medical needs. For a therapy to earn this privilege, it has to show a substantial advantage over existing treatments. Pelareorep, demonstrating nearly triple the average response rate compared to existing treatments, fits this bill impressively.
“This Fast Track designation is an important accomplishment that speaks to the impressive response rate and the durability of the response in our PDAC study. It also reflects the pressing need to improve upon the standard of care in this indication. We are excited for what’s ahead.”
– Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics Biotech Inc.
Indeed, this designation adds another feather in Oncolytics’ cap, marking a pivotal moment in their journey to bring forward a more effective treatment for pancreatic cancer. As the GOBLET study continues, the anticipation for more positive outcomes grows, and with it, the promise of pelareorep transforming the landscape of cancer treatment.
Looking Ahead
Pelareorep could be a significant step forward in the fight against pancreatic cancer. Its unique mode of operation could make it a critical weapon in the battle against this deadly disease. And it’s not just pancreatic cancer where pelareorep shows potential. The drug’s promise extends to other forms of cancer, too, such as breast cancer, which is another area of focus for Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC).
MEET THE EXPERTS AT ONCOLYTICS BIOTECH
Getting a new medical treatment like pelareorep ready for patients involves many steps and a skilled team. The experts at Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) are just the right people for the job. They have worked at leading companies and universities, such as Amgen, Bristol-Myers Squibb, and Harvard Medical School.
Let’s meet some of them:
Matt Coffey, PhD, MBA – Co-Founder, Director, President & CEO
Dr. Coffey is a cancer expert who has studied how a virus can help fight cancer. His research has been published in many notable scientific journals.
Thomas Heineman, MD, PhD – Head of Clinical Development and Operations
Dr. Heineman is a medical doctor who has spent over 25 years developing new drugs. He’s worked on projects for many types of cancer, including breast and pancreatic cancer, at places like GSK.
Andrew de Guttadauro – Global Head of Business Development
Mr. de Guttadauro has spent over 25 years helping new medical treatments reach patients. He has been a leader at big companies like Vical and Amgen.
Wayne Pisano, MBA – Chair of the Board
Pisano is an award-winning leader in the field of vaccines. He used to be the head of Sanofi Pasteur, one of the biggest vaccine companies in the world.
There are many other talented people at Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) too, from their other leaders to their Board of Directors and Scientific Advisory Board. They have worked at top companies and institutions like Ernst & Young LLP, Amgen, and Princeton University.
For example, Deborah M. Brown, B.Sc., M.B.A. is a Director who helps new medical businesses grow. She’s worked at EMD Serono and is now a leader at Accelera Canada.
Another Director, Bernd R. Seizinger, MD, PhD, is an expert in cancer drug discovery. He’s worked at Bristol-Myers Squibb and held top roles at Harvard Medical School and Princeton University. He’s now on the board of several biotech companies.
In short, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is guided by some of the best minds in the business.
RECAP: 8 Take Aways for Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC):
1. Top-Notch Collaborations
2. Better Response Rate
3. Complete Response Recorded
4. Fast Track Granted
5. Boosting Other Treatments
6. Exciting Data on the Horizon
7. Experienced Team
8. Well-Funded

BEFORE YOU GO CLICK AWAY!
You’ve made it this far, so it’s clear you’re interested. With big events on the horizon and the progress we’ve already seen, we think NOW IS THE RIGHT MOMENT for savvy folks to keep a close eye on Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC).
So, take some time to do your own research, and don’t forget to sign up for email alerts. That way, you’ll stay updated on all the exciting news and milestones from ONCY. We’re here to keep you informed!
USA News Group
Editorial Staff
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. USA News Group is a wholly-owned subsidiary of Market IQ Media Group, Inc. (“MIQ”). MIQ has been paid a fee for Oncolytics Biotech Inc. advertising and digital media from the company directly. There may be 3rd parties who may have shares of Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of MIQ own shares of Oncolytics Biotech Inc. which were purchased in the open market, and reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by MIQ has been approved by the above mentioned company; this is a paid advertisement, we currently own shares of Oncolytics Biotech Inc. and will buy and sell shares of the company in the open market, or through private placements, and/or other investment vehicles.
While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between the any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.
SOURCES CITED:
[1] https://www.whitehouse.gov/cancermoonshot/
[2] https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative
[3] https://www.pbs.org/newshour/politics/biden-to-seek-more-than-2-8-billion-from-congress-for-cancer-moonshot
[4] https://www.pfizer.com/news/press-release/press-release-detail/pfizer-invests-43-billion-battle-cancer
[5] https://www.businesswire.com/news/home/20230420005572/en/TPG-and-AmerisourceBergen-to-Acquire-Leading-Specialty-Practice-Network-OneOncology-From-General-Atlantic?
[6] https://www.fiercepharma.com/fierce-biotech-homepage/top-line-top-10-pharma-ma-deals-2022-and-what-amgens-purchase-horizon-means
[7] https://www.rbccm.com/en/gib/biopharma/episode/biotech-outlook-2023-small-is-the-new-big
[8] https://www.oncolyticsbiotech.com/press-releases/detail/588/oncolytics-biotech-receives-fda-fast-track-designation
[9] https://ir.oncolyticsbiotech.com/press-releases/detail/601/oncolytics-biotech-announces-updated-randomized-phase-2
[10] https://ir.oncolyticsbiotech.com/press-releases/detail/586/oncolytics-biotech-presents-updated-clinical-data-at-sitc
[11] https://www.nejm.org/doi/full/10.1056/nejmoa1304369
[12] https://www.precedenceresearch.com/immune-checkpoint-inhibitors-market#:~:text=The%20global%20immune%20checkpoint%20inhibitors,forecast%20period%202023%20to%202032
[13] JAMA Netw Open. 2019 May; 2(5): e192535
[14] https://www.gilead.com/news-and-press/press-room/press-releases/2016/5/kite-pharma-to-highlight-key-data-from-engineered-car-t-cell-therapy-pipeline-at-the-2016-american-society-of-clinical-oncology-annual-meeting
[15] https://www.investing.com/equities/kite-pharma-historical-data
[16] https://www.gilead.com/news-and-press/press-room/press-releases/2017/8/gilead-sciences-to-acquire-kite-pharma-for-119-billion
[17] https://www.prnewswire.com/news-releases/protagonist-therapeutics-to-present-updated-phase-2-rusfertide-clinical-results-in-polycythemia-vera-pv-at-asco-2022-301556326.html
[18] https://www.prnewswire.com/news-releases/arcellx-presents-continued-robust-long-term-responses-from-its-cart-ddbcma-phase-1-expansion-trial-in-patients-with-relapsed-or-refractory-multiple-myeloma-at-the-2022-asco-annual-meeting-301561200.html
[19] https://ir.oncolyticsbiotech.com/press-releases/detail/607/oncolytics-biotech-reports-second-quarter-2023-financial
[20] https://finance.yahoo.com/news/oncolytics-biotech-successfully-raises-us-125000351.html
[21] https://seer.cancer.gov/statfacts/html/pancreas.html
[22] Includes U.S., Major European Markets, and Japan; Source: 2020 Pancreatic Cancer Disease Landscape & Forecast, (Clarivate, 2020)
[23] Von Hoff D et al. N Engl J Med 2013; 369:1691-1703 DOI: 10.1056/NEJMoa1304369
[24] O’Reilly et al. Eur J Cancer. 2020 June ; 132: 112–121. DOI:10.1016/j.ejca.2020.03.005
[25] Karasic et al. JAMA Oncol. 2019 Jul 1;5(7):993- 998. DOI: 10.1001/jamaoncol.2019.0684
[26] Tempero et al. Ann Oncol. 2021 May;32(5):600-608. DOI: 10.1016/j.annonc.2021.01.070
[27] Luchini C, Brosens LAA, Wood LD, et al. Gut 2021;70:148–156
[28] https://finance.yahoo.com/news/oncolytics-biotechs-pelareorep-selected-inclusion-110000604.html
[29] https://www.oncolyticsbiotech.com/press-releases/press-releases/detail/602/oncolytics-biotechs-pelareorep-selected-for-inclusion-in
[30] https://www.oncolyticsbiotech.com/press-releases/press-releases/detail/588/oncolytics-biotech-receives-fda-fast-track-designation
[31] https://www.oncolyticsbiotech.com/press-releases/press-releases/detail/586/oncolytics-biotech-presents-updated-clinical-data-at-sitc


