Discover the Hidden Gem in Biotech: Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC). After an Astounding Surge in Patient Response Rate in 2022’s GOBLET Study and Earning an FDA Fast Track Designation, and Are Coming Off a Recent Rare Honour at the 2023 ASCO Annual Meeting—an Oral Presentation Slot. With Wall Street Analysts Touting a Universal STRONG BUY Rating, and Eyeing a Potential $15.00 Price Target (Currently at $1.91)—That’s a Potential Increase of over 685%! Don’t Miss this Chance to Explore this Rising Star in Cancer Research. Get Ahead of this News TODAY!
EDITOR’S NOTE* With some Wall Street analyst coverage giving ONCY a price target of $15.00, these rock-bottom prices you are currently seeing probably won’t last long – An earlier publication of this report saw a potential increase of +685% from its current levels, prior to the beginning of what we believe will be a series of catalyst events on the horizon. NOW they’ve already begun with the FDA’s Fast Track Designation for pelareorep with pancreatic cancer. In the first day of trading after the Fast Track approval announcement, shares rose by 25%, showing that time is running out to get early positioning, so get positioned now! Take a look here: https://www.nasdaq.com/market-activity/stocks/oncy/analyst-research
According to analysts at RBC Capital Markets, “Small is the New Big” in terms of their outlook for the Biotech Sector in 2023.[1] In their Biotech Outlook 2023 report, they state: “Small-cap firms may be primed to outperform in the year ahead, in part due to a more permissive regulatory environment.”
Their expectation is for even better performance for higher quality, catalyst-driven small and mid-cap companies as 2023 progresses.
So… what does the smart investor look for amid such a field? Why is there STILL hesitation towards investing in the Biotech Sector? What they’re looking for are CATALYSTS!
And while it’s easy to identify catalysts tied to the FDA’s expedited types of approvals (fast track, breakthrough, etc.), there’s another even rarer catalyst event that’s usually reserved only for multibillion-dollar giants…
That event is the ASCO Annual Meeting, the biggest and most prestigious conference in the cancer research world. In particular, it’s those who are selected to give an ORAL PRESENTATION that garners the most attention by the time the event comes to a close.
At this year’s 2023 ASCO Annual Meeting, one company, in particular, has data in hand that piqued the ASCO selection committee’s interest enough to be worthy of an oral presentation—often reserved for a small select few, and typically the multibillion-dollar pharma giants.
That company is Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC), a cutting-edge development-stage biopharmaceutical company, with a lead product called pelareorep designed to treat solid tumors and hematological malignancies, and several co-development agreements in place, including with Merck KGaA and Pfizer Inc., that’s positioned to become a game changer in the cancer space.
At ASCO, companies from all over the world gather to present their latest cancer-fighting therapies, and the stakes are high. The right presentation at ASCO can be a game-changer for a company, and it can make their stock price skyrocket.
For example, the case of Kite Pharma, which gave an oral presentation at the 2016 ASCO annual meeting on June 6, 2016,[2] setting their share price on a journey from $53.72 through an $11.9 billion takeover by Gilead Sciences (NASDAQ:GILD)—announced just over a year later[3]—on to its last share price of $179.79 (a gain of 234%).[4]
Last year in 2022, Protagonist Therapeutics, Inc. (NASDAQ:PTGX) gave an oral presentation on June 7, 2022, where their share price opened on that day at $8.81 and went on a run to its current price of $24.45[5]—an increase of 177.5%.
Even better was the story of Arcellx, Inc. (NASDAQ:ACLX), which presented clinical results during an oral presentation at the ASCO Annual Meeting on June 5, 2022[6] where it opened at $13.30 on its way to its current share price of $43.50—an increase of over 227% in less than a year.
Which brings us back to the upcoming oral presentation at the ASCO 2023 Annual Meeting for Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC).
Oncolytics Biotech is a cutting-edge biotech company that’s been working on a new way to fight cancer. They’re using the immune system to attack tumors, which is a really innovative approach.
EXPERTS are giving Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) a STRONG BUY rating, as per NASDAQ.com’s consensus of Analyst ratings.

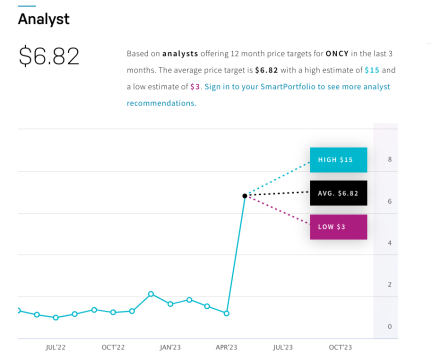
The average price target for each of these analysts is $6.82 with a high estimate of $15—while the current stock price as of May 4, 2023 is just $1.45.
That’s a growth projection of nearly 10x its current price of $1.45 per share!
An increase of 934%!
 What could these analysts be seeing that the rest of the market is not? Well, we can’t speak for them, but we can tell you what we’re seeing and why ONCY is one of the best gems we’ve come across in years.
What could these analysts be seeing that the rest of the market is not? Well, we can’t speak for them, but we can tell you what we’re seeing and why ONCY is one of the best gems we’ve come across in years.
Now YOU TOO can look hard enough at the small amounts of data that’s already available, and see what we see… ONCY is an absolute STEAL in the making!
So let’s now do a deep dive on the Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) story, and why NOW is the perfect time for investors to give them more of your attention.
8 Reasons Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) and Pelareorep are Positioned as an Oncology Game Changer
1 Pelareorep’s Big League Collaborations, Partnerships, and Combos: Pelareorep’s potential has drawn a partnership with Adlai Nortye, co-development with Pfizer and Merck KGaA, as well as collaborations with SOLTI and Roche, as well as combinations in developments with Merck & Co., Bristol-Myers Squibb, and Incyte.
2 Near TRIPLING of Objective Response Rate (ORR): A historical control trial by Dr. Von Hoff titled Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine yielded only about 25% ORR[7], while Oncolytics Biotech’s flagship pelareorep’s ORR was reported at 69% in the pancreatic cancer cohort with patients achieving a complete or partial response.[8]
3 Achieved a Confirmed COMPLETE Response (CR): Also in the most recent results, pelareorep achieved a confirmed CR, which is almost unheard of, given that pelareorep achieved this with one of thirteen evaluable patients, while patients in the Von Hoff trial only achieved one CR from a total of 861 patients—that’s a 7.7% rate vs a 0.12% rate, or a +6,500% (or 65x) improvement over the previous industry benchmark so far.
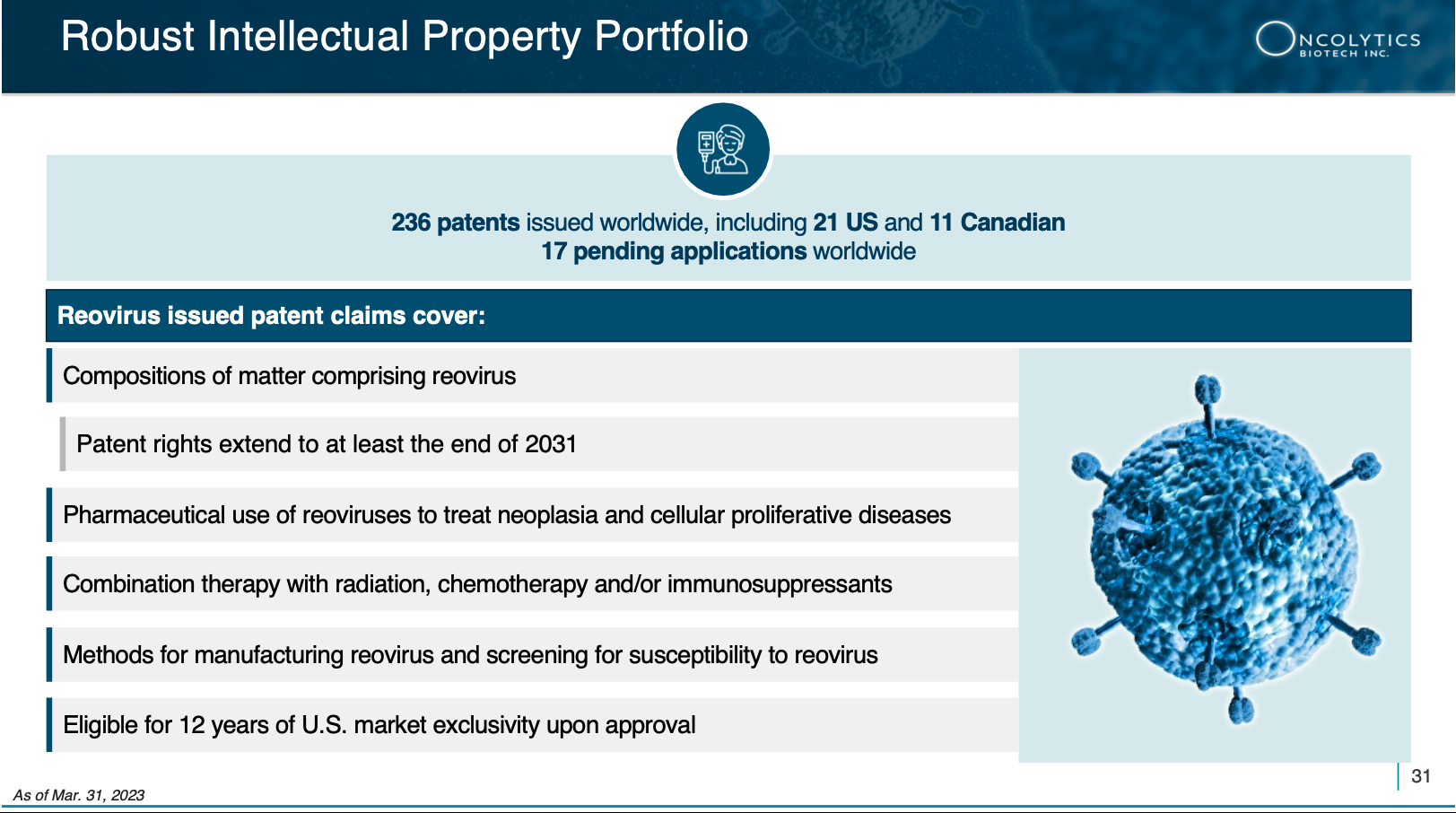
4 Fast Track Designation GRANTED: On December 1, 2022, Oncolytics Biotech announced that the FDA granted Fast Track Designation to pelareorep for use in combination with Roche’s anti-PD-L1 checkpoint inhibitor atezolizumab, and the chemotherapeutic agents gemcitabine and nab-paclitaxel, for the treatment of advanced/metastatic pancreatic ductal adenocarcinoma (PDAC).
5 Synergy with Immune Checkpoint Inhibitors (ICIs): The ICI market is expected to exceed $55 billion by 2025[9], despite as few as 1 in 5 patients responding to ICI therapy[10]. Pelareorep has clinically demonstrated its ability to synergize with these ICIs, perhaps enhancing their effectiveness.
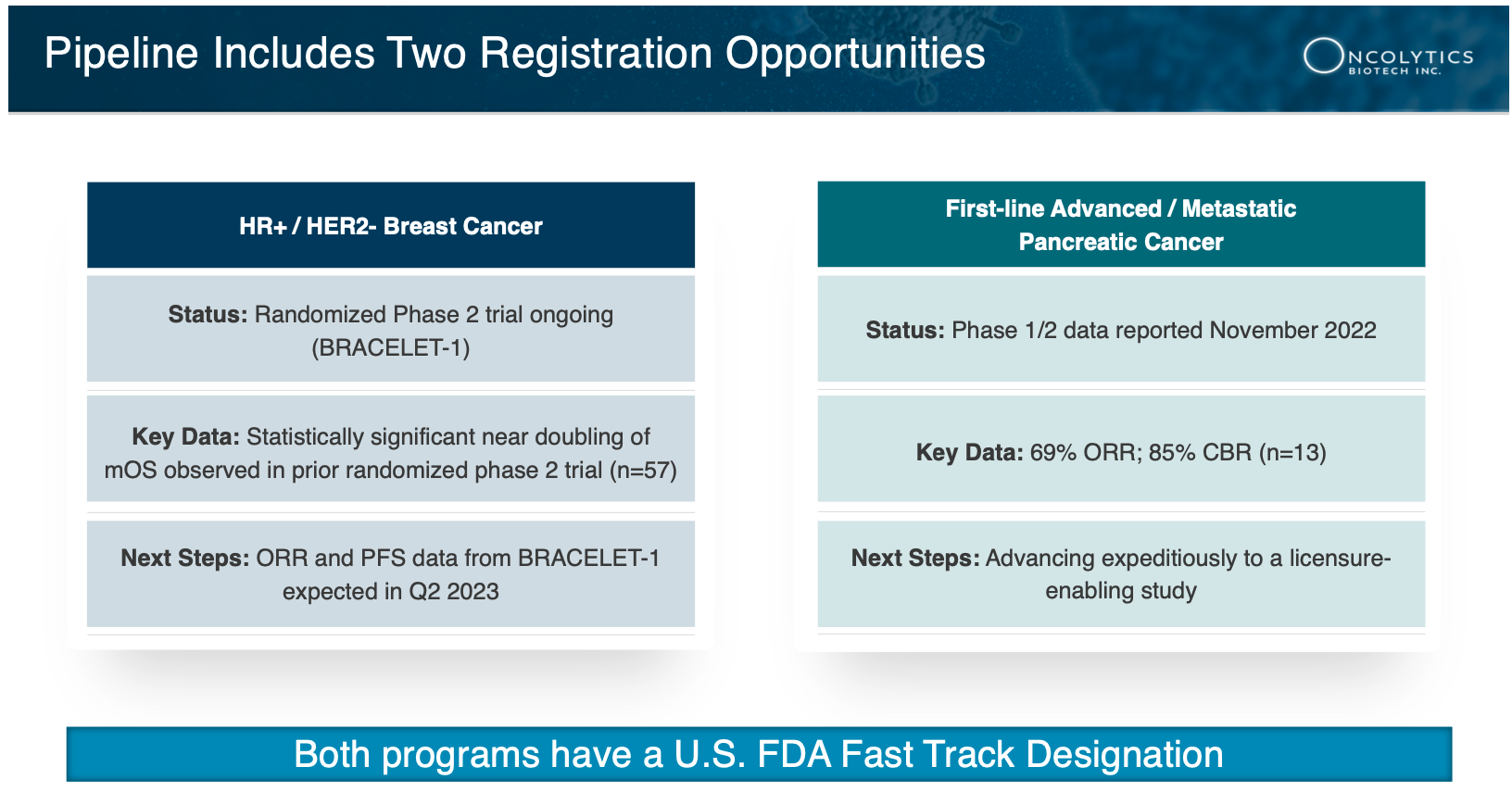
6 More Important Data to Come: Not only did Oncolytics Biotech present incredible data from its GOBLET study that led to its Fast Track designation, but there is also an upcoming catalyst expected in the first half of 2023 from its Phase 2 trial in HR+/HER2- breast cancer to facilitate the asset’s advancement to a registrational study. Now as ASCO 2023 approaches in June, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is set to give an oral presentation delivering data from its randomized BRACELET-1 trial in HR+/HER2- metastatic breast cancer, offering another major potential catalyst event.
7 Strong Leadership Team: Proven Management Team and Board of Directors that combines over 150 years of experience in drug development and the biopharmaceutical industry, including a World Congress Pharma Executive of the Year award winner Chair of the Board.
8 Fully-Financed Through 2023: As of March 31, 2023, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) has C$29.7 million (US$22.2M) in cash and cash equivalents, which provides projected runway through key milestones beyond March 2024.
BREAST & PANC: TWO LEGIT PATHS TO FDA APPROVAL
Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) has been clear in its objectives to target important segments, beginning with breast cancer, which is projected to reach US$55.27 billion by 2027, growing at a CAGR of 13.1% along the way.[11]
At this year’s ASCO Annual Meeting, ONCY is set to deliver data from its randomized BRACELET-1 trial in HR+/HER2- metastatic breast cancer. Though the market doesn’t know what the data will entail, obviously representatives from ASCO have seen enough to warrant giving a small-cap biotech company a chance to give an oral presentation rather than just a poster presentation at the event—which is an extremely rare honor to be given to a company with a market cap of approximately $100 million.
However, that’s not the ONLY form of cancer that pelareorep is targeting. Don’t discount pelareorep’s FDA Fast Track Designation approval[12] to treat pancreatic cancer.
With a 92% mortality rate, pancreatic cancer is a nearly automatic death sentence.[13] Once pancreatic cancer has spread to other organs, surrounding lymph nodes, or other parts of the body, the average life expectancy is just 3-6 months.[14]
In comparison, breast cancer diagnoses carry with them an average 91% 5-year survival rate across all SEER stages.[15]
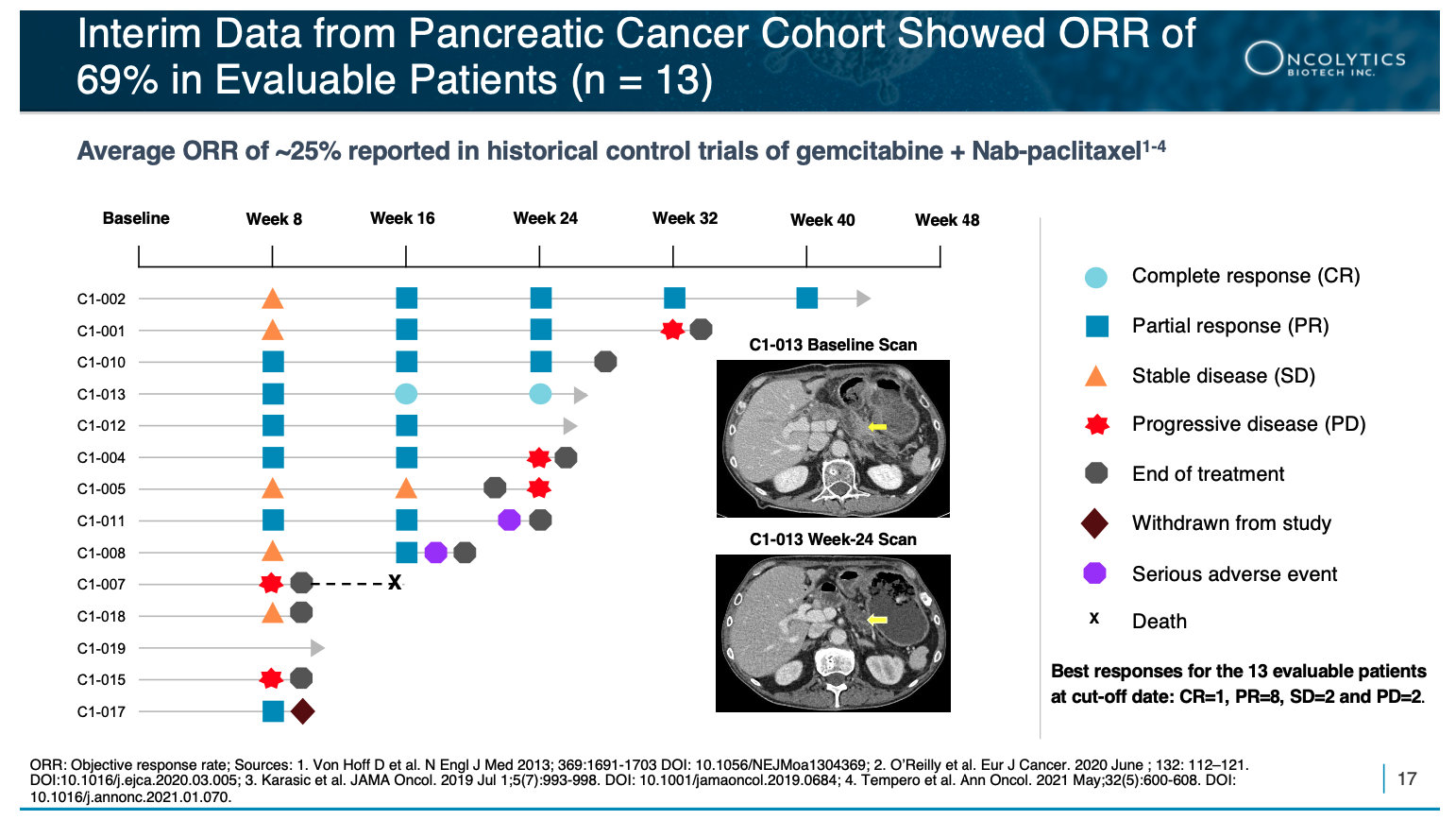
THE BIG WIN IN PANCREATIC CANCER
Through the Phase 1/2 GOBLET study, Oncolytics Biotech Inc.’s (NASDAQ:ONCY) (TSX:ONC) pelareorep demonstrated a 69% Objective Response Rate (ORR) in pancreatic cancer[16], which was remarkable, given it delivered triple the average ORR seen in a historical control trial of the standard of care chemotherapy, which was only ~25%.[17]
Pelareorep also achieved a Complete Response (CR), which in pancreatic cancer is almost UNHEARD OF. In a large historical control trial, there was a single CR in 861 patients, whereas this new therapy achieved a CR in just 13 patients—marking a significant potential 65x improvement over historical results!
Prior to the GOBLET study, the control benchmarks for success in pancreatic cancer came in the 2013 reference in the New England Journal of Medicine by Dr. Von Hoff, titled Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine.[18]
Why this study?
Because it fulfills the role of a historical control trial involving gemcitabine (gemzar) (Eli Lilly) plus nab-paclitaxel (Bristol-Myers Squibb).
How were the 2013 study’s results?
- The median overall survival (OS) was 8.5 months for the combo vs 6.7 months for gemcitabine alone
- The survival rate was 35% in the combo group vs 22% in the gemcitabine group alone at 1 year
- 9% vs 4% at 2 years
- The median progression-free survival (PFS) was 5.5 months in the combo vs 3.7 months in the gemcitabine only group.
- The response rate was 23% vs 7%
- 1 in 861 (0.12%) patients achieved a Complete Response (CR)
Those results were good enough to see the FDA approve nab-paclitaxel for use in September of 2013.[19]
To recap, before it was approved, nab-paclitaxel:
- Increased mean overall survival (OS) duration by 26.8%
- Increased median progression-free survival (PFS) duration by 48.6%
- Increased 1-year survival rate by 59%
- Increased 2-year survival rate by 125%
- Increased response rate by 228.5%
Now, the GOBLET study is evaluating pelareorep in combination with Roche’s anti-PD-L1 checkpoint inhibitor atezolizumab and gemcitabine + nab-paclitaxel.
In late June 2022, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) announced it had achieved success criteria for efficacy in the study’s pancreatic cancer cohort.[20]
NOW… we have the updated results from the phase 1/2 GOBLET study’s first-line advanced/metastatic pancreatic ductal adenocarcinoma (PDAC) cohort.
And they… were… FANTASTIC!
- 1 in 13 (7.7%) evaluable patients achieved a confirmed Complete Response (CR)
- 8 of 13 (61.5%) evaluable patients achieved a PR
- 2 of 13 (15.4%) evaluable patients achieved stable disease (SD)
- 9 of 13 (69.2%) evaluable patients achieved a response
- The observed ORR of 69% is substantially higher than the average ORR of ~25% reported in historical control trials of gemcitabine and nab-paclitaxel in pancreatic cancer.[21],[22],[23],[24]
- GOBLET’s PDAC cohort exceeded the protocol-specified success criterion for Stage 1 of ≥ 3/12 objective responses
- The studied treatment combination has been well tolerated, with no safety concerns identified to date
A copy of the poster is available on the Posters & Publications page of Oncolytics’ website (LINK).
BIG RESULTS!: On December 1, 2022, the FDA granted Fast Track Designation to pelareorep in combination with Roche’s anti-PD-L1 checkpoint inhibitor atezolizumab, and the chemotherapeutic agents gemcitabine and nab-paclitaxel, for the treatment of advanced/metastatic pancreatic ductal adenocarcinoma (PDAC).
“Receiving this Fast Track designation is an important accomplishment that speaks to the impressive response rate and the durability of the response in our PDAC study, and it also reflects the pressing need to improve upon the standard of care in this indication. With our core programs in breast and pancreatic cancer both nearing pivotal trials, and eligible for the Fast Track program’s numerous benefits, we believe we are at a crucial point in Oncolytics’ evolution and are excited for what’s ahead.”
Matt Coffey, President and Chief Executive Officer of Oncolytics Biotech Inc.
A CLOSER LOOK AT PELAREOREP
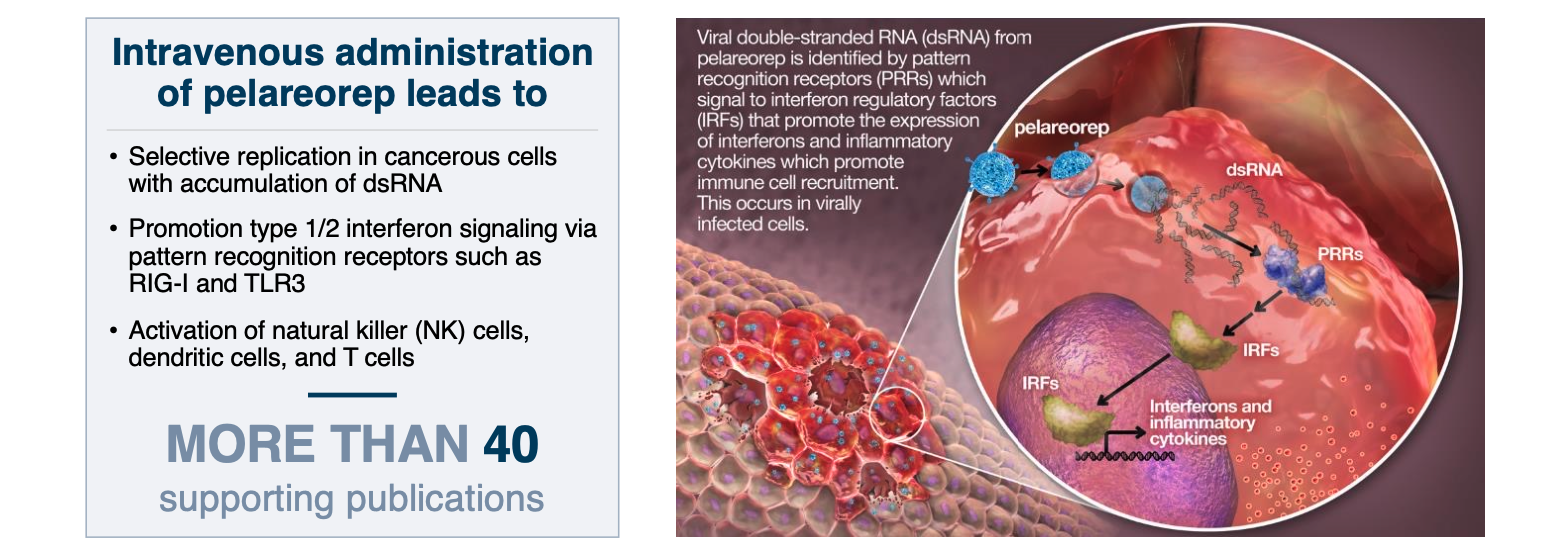 FRIENDS IN HIGH PLACES
FRIENDS IN HIGH PLACES
Oncolytics has established a successful partnership with Adlai Nortye in China, Hong Kong, Macau, Singapore, South Korea and Taiwan.
Perhaps most notable are the studies Oncolytics has performed or is performing with Pfizer, Merck KGaA, Merck, Bristol-Myers Squibb, Incyte, and Roche. These involve chemotherapies and/or checkpoint inhibitors, targeting metastatic breast cancer, early-stage breast cancer, multiple myeloma and pancreatic cancer.
 THE BRAINTRUST BEHIND ONCOLYTICS BIOTECH
THE BRAINTRUST BEHIND ONCOLYTICS BIOTECH
In order to take a proprietary biotech asset such as pelareorep through the trials along the road to approval, the entire process requires good stewardship. Thankfully, Oncolytics Biotech Inc. (NASDAQ:ONCY) (TSX:ONC) is in VERY capable hands, built upon experience among management and directors with several leaders in the sector such as Amgen, Bristol-Myers Squibb, GSK, Sanofi Pasteur, National Cancer Institute-Frederick Cancer Research and Development Center, Harvard Medical School, and Princeton University.
ONCY’s leadership team includes:
Co-Founder, Director, President & CEO – Matt Coffey, PhD, MBA
Dr. Coffey completed his doctorate degree in oncology at the University of Calgary with a focus on the oncolytic capabilities of the reovirus. The results from his research have been published in various respected scientific journals, including Science, Human Gene Therapy, and The EMBO Journal.
Head of Clinical Development and Operations – Thomas Heineman, MD, PhD
Dr. Heineman completed his medical degree and doctorate in Virology at the University of Chicago. His drug development experience spans more than 25 years, including 15 years in the biopharmaceutical industry. He has held senior roles at GSK, were he led the clinical development of Shingrix, and at several biotechnology companies where his focus has been oncology and immuno-oncology. Dr. Heineman has led clinical programs in multiple oncology indications, including breast cancer, pancreatic cancer, B-cell lymphoma, glioblastoma and colorectal cancer.
Global Head of Business Development – Andrew de Guttadauro
de Guttadauro is +25-year biopharmaceutical commercialization and business development veteran who’s held executive and senior-level positions at leading pharmaceutical and biotechnology companies, including as VP of Corporate Development at Vical, supporting the execution of distribution agreements for Allovectin® and a variety of positions at Amgen where he contributed to the success of Enbrel®, Aranesp®, and Epogen® before joining MedImmune to lead marketing efforts for the FluMist® inhaled influenza vaccine. He also served as Director of Strategy at Biogen Idec.
Chair of the Board – Wayne Pisano, MBA
Pisano was recognized as Pharma Executive of the Year by the World Vaccine Congress in 2010. He served as the President and CEO of VaxInnate and has been a Board Member of Immunovaccine since 2011. He is the former President and CEO of Sanofi Pasteur, one of the largest vaccine companies in the world. He’s credited with driving SanofiPasteur’s leadership within the worldwide influenza market and capturing 50% of global sales.
The remaining leadership roles, Board of Directors, and Scientific Advisory Board consist of highly qualified members, with senior level experience at such companies and institutions as: Ernst & Young LLP, Nabisco, Hospital for Sick Children, Aptose Biosciences, Achillion Pharmaceuticals, National Cancer Institute-Frederick Cancer Research and Development Center, GPC Biotech, Harvard Medical School, Princeton University, Massachusetts General Hospital, EMD Serono, Breast International Group (BIG), SOLTI – Breast Cancer Research Group, Amgen & BMS IO Network and more.
Director – Deborah M. Brown, B.Sc., M.B.A.
Brown is currently a Managing Partner at Accelera Canada, a specialty consultancy firm that assists emerging biopharma ventures in the United States and Europe with the development and implementation of Canadian market strategies. She held progressively senior roles at EMD Serono from 2000 to 2014, including Executive Vice President of Neuroimmunology for the company’s U.S. operations, and President and Managing Director of the company’s Canadian operations. In 2012, Brown was Chair of the National Pharmaceutical Organization (now Innovative Medicines Canada) and served on its Board of Directors from 2007 to 2014. She currently sits on the Boards of Life Sciences Ontario, the Strategic Executive Advisory Council for Canadian Cancer Trials Group, and her local SPCA.
Director – Bernd R. Seizinger, MD, PhD
Dr. Seizinger currently serves as chairman/board member in a number of public and private biotech companies in the U.S., Europe and Canada, including: Vaccibody, Oxford BioTherapeutics, Aprea, CryptoMedix, and BioInvent. Previously he was President & CEO of public oncology company GPC Biotech; VP Oncology Drug Discovery and – in parallel – VP Corporate and Academic Alliances at Bristol-Myers Squibb; and Executive VP and CSO, Genome Therapeutics. Prior to his corporate appointments, he held Senior Faculty positions at Harvard Medical School, Massachusetts General Hospital and Princeton University.
RECAP: 8 IMPORTANT POINTS TO REMEMBER FOR ONCOLYTICS BIOTECH INC. (NASDAQ:ONCY) (TSX:ONC)
1. SIGNIFICANT COLLABORATIONS
2. NEAR TRIPLING OF ORR / NEAR DOUBLING OF OS
3. CONFIRMED COMPLETE RESPONSE
4. FAST TRACK DESIGNATION GRANTED
5. SYNERGY W/ MULTIPLE ONCOLOGY TREATMENTS
6. MORE DATA TO COME
7. STRONG LEADERSHIP TEAM
8. FULLY-FINANCED THROUGH 2023
Now that you’ve read this far, the case has been made strong enough for you to get into action.
With what we believe are catalysts coming in the very near future, and with the data we’ve already seen, we believe THIS IS THE PERFECT TIME for smart investors to seriously follow the ongoing ONCOLYTICS BIOTECH INC. (NASDAQ:ONCY) (TSX:ONC) story.
So, do your own due diligence, and don’t forget to click here to sign up for email alerts to make sure you don’t miss out on any of ONCY’s news and milestones.
USA News Group
Editorial Staff
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. USA News Group is a wholly-owned subsidiary of Market IQ Media Group, Inc. (“MIQ”). MIQ has been paid a fee for Oncolytics Biotech Inc. advertising and digital media from the company directly. There may be 3rd parties who may have shares of Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of MIQ own shares of Oncolytics Biotech Inc. which were purchased in the open market, and reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by MIQ has been approved by the above mentioned company; this is a paid advertisement, we currently own shares of Oncolytics Biotech Inc. and will buy and sell shares of the company in the open market, or through private placements, and/or other investment vehicles.
While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between the any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.
SOURCES CITED
[1] https://www.rbccm.com/en/gib/biopharma/episode/biotech-outlook-2023-small-is-the-new-big
[2] https://www.gilead.com/news-and-press/press-room/press-releases/2016/5/kite-pharma-to-highlight-key-data-from-engineered-car-t-cell-therapy-pipeline-at-the-2016-american-society-of-clinical-oncology-annual-meeting
[3] https://www.gilead.com/news-and-press/press-room/press-releases/2017/8/gilead-sciences-to-acquire-kite-pharma-for-119-billion
[4] https://www.investing.com/equities/kite-pharma-historical-data
[5] https://www.prnewswire.com/news-releases/protagonist-therapeutics-to-present-updated-phase-2-rusfertide-clinical-results-in-polycythemia-vera-pv-at-asco-2022-301556326.html
[6] https://www.prnewswire.com/news-releases/arcellx-presents-continued-robust-long-term-responses-from-its-cart-ddbcma-phase-1-expansion-trial-in-patients-with-relapsed-or-refractory-multiple-myeloma-at-the-2022-asco-annual-meeting-301561200.html
[7] https://www.nejm.org/doi/full/10.1056/nejmoa1304369
[8] https://ir.oncolyticsbiotech.com/press-releases/detail/586/oncolytics-biotech-presents-updated-clinical-data-at-sitc
[9] Cowen and Company, LLC, “Therapeutic Categories Outlook,” February 2021;
[10] JAMA Netw Open. 2019 May; 2(5): e192535
[11] https://www.fortunebusinessinsights.com/industry-reports/breast-cancer-therapeutics-market-100163
[12] https://finance.yahoo.com/news/oncolytics-biotech-receives-fda-fast-123200369.html
[13] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9277424/
[14] https://www.griswoldhomecare.com/blog/2016/november/pancreatic-cancer-life-expectancy-what-to-expect/#:~:text=Once%20pancreatic%20cancer%20has%20spread,just%20three%20to%20six%20months.
[15] https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html
[16] https://ir.oncolyticsbiotech.com/press-releases/detail/586/oncolytics-biotech-presents-updated-clinical-data-at-sitc
[17] https://www.nejm.org/doi/full/10.1056/nejmoa1304369
[18] https://www.nejm.org/doi/full/10.1056/nejmoa1304369
[19] https://www.cancer.gov/types/pancreatic/research/nab-paclitaxel-gemcitabine
[20] https://ir.oncolyticsbiotech.com/press-releases/detail/578/oncolytics-biotech-achieves-success-criteria-for-efficacy
[21] Von Hoff D et al. N Engl J Med 2013; 369:1691-1703 DOI: 10.1056/NEJMoa1304369
[22] O’Reilly et al. Eur J Cancer. 2020 June; 132: 112-121. DOI:10.1016/j.ejca.2020.03.005
[23] Karasic et al. JAMA Oncol. 2019 Jul 1; 5(7):993-998. DOI: 10.1001/jamaoncol.2019.0684
[24] Tempero et al. Ann Oncol. 2021 May; 32(5):600-608. DOI: 10.1016/j.annonc.2021.01.070